| Sympathetic nervous system | |
|---|---|

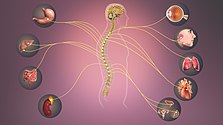
Schematic illustration showing the sympathetic nervous system with sympathetic cord and target organs.
| |
| Details | |
| Identifiers | |
| Latin | pars sympathica divisionis autonomici systematis nervosi |
| Acronym(s) | SNS |
| MeSH | D013564 |
| TA | A14.3.01.001 |
| FMA | 9906 |
The sympathetic nervous system (SNS) is one of the two main divisions of the autonomic nervous system, the other being the parasympathetic nervous system. (The enteric nervous system (ENS) is now usually referred to as separate from the autonomic nervous system since it has its own independent reflex activity.)
The autonomic nervous system functions to regulate the body's unconscious actions. The sympathetic nervous system's primary process is to stimulate the body's fight-flight-or-freeze response. It is, however, constantly active at a basic level to maintain homeostasis homeodynamics. The sympathetic nervous system is described as being antagonistic to the parasympathetic nervous system which stimulates the body to "feed and breed" and to (then) "rest-and-digest".
Structure
There are two kinds of neurons involved in the transmission of any signal through the sympathetic system: pre-ganglionic and post-ganglionic. The shorter preganglionic neurons originate in the thoracolumbar division of the spinal cord specifically at T1 to L2~L3, and travel to a ganglion, often one of the paravertebral ganglia, where they synapse with a postganglionic neuron. From there, the long postganglionic neurons extend across most of the body.
At the synapses within the ganglia, preganglionic neurons release acetylcholine, a neurotransmitter that activates nicotinic acetylcholine receptors on postganglionic neurons. In response to this stimulus, the postganglionic neurons release norepinephrine, which activates adrenergic receptors
that are present on the peripheral target tissues. The activation of
target tissue receptors causes the effects associated with the
sympathetic system. However, there are three important exceptions:
- Postganglionic neurons of sweat glands release acetylcholine for the activation of muscarinic receptors, except for areas of thick skin, the palms and the plantar surfaces of the feet, where norepinephrine is released and acts on adrenergic receptors.
- Chromaffin cells of the adrenal medulla are analogous to post-ganglionic neurons; the adrenal medulla develops in tandem with the sympathetic nervous system and acts as a modified sympathetic ganglion. Within this endocrine gland, pre-ganglionic neurons synapse with chromaffin cells, triggering the release of two transmitters: a small proportion of norepinephrine, and more substantially, epinephrine. The synthesis and release of epinephrine as opposed to norepinephrine is another distinguishing feature of chromaffin cells compared to postganglionic sympathetic neurons.
- Postganglionic sympathetic nerves terminating in the kidney release dopamine, which acts on dopamine D1 receptors of blood vessels to control how much blood the kidney filters. Dopamine is the immediate metabolic precursor to norepinephrine, but is nonetheless a distinct signaling molecule.
Organization
The sympathetic nervous system extends from the thoracic to lumbar vertebrae and has connections with the thoracic, abdominal, and pelvic plexuses.
Sympathetic nerves arise from near the middle of the spinal cord in the intermediolateral nucleus of the lateral grey column, beginning at the first thoracic vertebra of the vertebral column and are thought to extend to the second or third lumbar
vertebra. Because its cells begin in the thoracolumbar division – the
thoracic and lumbar regions of the spinal cord, the sympathetic nervous
system is said to have a thoracolumbar outflow. Axons of these nerves leave the spinal cord through the anterior root.
They pass near the spinal (sensory) ganglion, where they enter the
anterior rami of the spinal nerves. However, unlike somatic innervation,
they quickly separate out through white rami connectors (so called from the shiny white sheaths of myelin
around each axon) that connect to either the paravertebral (which lie
near the vertebral column) or prevertebral (which lie near the aortic
bifurcation) ganglia extending alongside the spinal column.
To reach target organs and glands, the axons must travel long
distances in the body, and, to accomplish this, many axons relay their
message to a second cell through synaptic transmission. The ends of the axons link across a space, the synapse, to the dendrites of the second cell. The first cell (the presynaptic cell) sends a neurotransmitter
across the synaptic cleft where it activates the second cell (the
postsynaptic cell). The message is then carried to the final
destination.
Presynaptic nerves' axons terminate in either the paravertebral ganglia or prevertebral ganglia.
There are four different paths an axon can take before reaching its
terminal. In all cases, the axon enters the paravertebral ganglion at
the level of its originating spinal nerve. After this, it can then
either synapse in this ganglion, ascend to a more superior or descend to
a more inferior paravertebral ganglion and synapse there, or it can
descend to a prevertebral ganglion and synapse there with the
postsynaptic cell.
The postsynaptic cell then goes on to innervate the targeted end
effector (i.e. gland, smooth muscle, etc.). Because paravertebral and
prevertebral ganglia are relatively close to the spinal cord,
presynaptic neurons are generally much shorter than their postsynaptic
counterparts, which must extend throughout the body to reach their
destinations.
A notable exception to the routes mentioned above is the
sympathetic innervation of the suprarenal (adrenal) medulla. In this
case, presynaptic neurons pass through paravertebral ganglia, on through
prevertebral ganglia and then synapse directly with suprarenal tissue.
This tissue consists of cells that have pseudo-neuron like qualities in
that when activated by the presynaptic neuron, they will release their
neurotransmitter (epinephrine) directly into the bloodstream.
In the sympathetic nervous system and other components of the
peripheral nervous system, these synapses are made at sites called
ganglia. The cell that sends its fiber is called a preganglionic cell,
while the cell whose fiber leaves the ganglion is called a postganglionic
cell. As mentioned previously, the preganglionic cells of the
sympathetic nervous system are located between the first thoracic
segment and third lumbar segments of the spinal cord. Postganglionic
cells have their cell bodies in the ganglia and send their axons to
target organs or glands.
The ganglia include not just the sympathetic trunks but also the cervical ganglia (superior, middle and inferior), which send sympathetic nerve fibers to the head and thorax organs, and the celiac and mesenteric ganglia, which send sympathetic fibers to the gut.
| Organ | Nerves | Spinal column origin |
|---|---|---|
| stomach | T5, T6, T7, T8, T9, sometimes T10 | |
| duodenum | T5, T6, T7, T8, T9, sometimes T10 | |
| jejunum and ileum | T5, T6, T7, T8, T9 | |
| spleen | T6, T7, T8 | |
| gallbladder and liver |
|
T6, T7, T8, T9 |
| colon |
| |
| pancreatic head | T8, T9 | |
| appendix |
|
T10 |
| kidneys and ureters |
|
T11, T12 |
Information transmission
Sympathetic Nervous System - Information transmits through it affecting various organs.
Messages travel through the sympathetic nervous system in a bi-directional flow. Efferent
messages can trigger changes in different parts of the body
simultaneously. For example, the sympathetic nervous system can
accelerate heart rate; widen bronchial passages; decrease motility (movement) of the large intestine; constrict blood vessels; increase peristalsis in the oesophagus; cause pupillary dilation, piloerection (goose bumps) and perspiration (sweating);
and raise blood pressure. One exception is with certain blood vessels
such as those in the cerebral and coronary arteries, which dilate
(rather than constrict) with an increase in sympathetic tone. This is
because of a proportional increase in the presence of β2 adrenergic receptors rather than α1 receptors. β2
receptors promote vessel dilation instead of constriction like α1
receptors. An alternative explanation is that the primary (and direct)
effect of sympathetic stimulation on coronary arteries is
vasoconstriction followed by a secondary vasodilation caused by the
release of vasodilatory metabolites due to the sympathetically increased
cardiac inotropy and heart rate. This secondary vasodilation caused by
the primary vasoconstriction is termed functional sympatholysis, the
overall effect of which on coronary arteries is dilation.
The target synapse of the postganglionic neuron is mediated by adrenergic receptors and is activated by either norepinephrine (noradrenaline) or epinephrine (adrenaline).
Function
| Organ | Effect |
|---|---|
| Eye | Dilates |
| Heart | Increases rate and force of contraction |
| Lungs | Dilates bronchioles via circulating adrenaline |
| Blood vessels | Dilate in skeletal muscle |
| Digestive system | Constricts in gastrointestinal organs |
| Sweat glands | Activates sweat secretion |
| Digestive tract | Inhibits peristalsis |
| Kidney | Increases renin secretion |
| Penis | Inhibits tumescence |
| Ductus deferens | Promotes emission prior to ejaculation |
The sympathetic nervous system is responsible for up- and
down-regulating many homeostatic mechanisms in living organisms. Fibers
from the SNS innervate tissues in almost every organ system, providing
at least some regulation of functions as diverse as pupil diameter, gut motility, and urinary system output and function. It is perhaps best known for mediating the neuronal and hormonal stress response commonly known as the fight-or-flight response. This response is also known as sympatho-adrenal response of the body, as the preganglionic sympathetic fibers that end in the adrenal medulla
(but also all other sympathetic fibers) secrete acetylcholine, which
activates the great secretion of adrenaline (epinephrine) and to a
lesser extent noradrenaline (norepinephrine) from it. Therefore, this
response that acts primarily on the cardiovascular system is mediated directly via impulses transmitted through the sympathetic nervous system and indirectly via catecholamines secreted from the adrenal medulla.
The sympathetic nervous system is responsible for priming the body for action, particularly in situations threatening survival.
One example of this priming is in the moments before waking, in which
sympathetic outflow spontaneously increases in preparation for action.
Sympathetic nervous system stimulation causes vasoconstriction of
most blood vessels, including many of those in the skin, the digestive
tract, and the kidneys. This occurs as a result of activation of alpha-1
adrenergic receptors by norepinephrine released by post-ganglionic
sympathetic neurons. These receptors exist throughout the vasculature of
the body but are inhibited and counterbalanced by beta-2 adrenergic
receptors (stimulated by epinephrine release from the adrenal glands) in
the skeletal muscles, the heart, the lungs, and the brain during a
sympathoadrenal response. The net effect of this is a shunting of blood
away from the organs not necessary to the immediate survival of the
organism and an increase in blood flow to those organs involved in
intense physical activity.
Sensation
The afferent fibers of the autonomic nervous system,
which transmit sensory information from the internal organs of the body
back to the central nervous system (or CNS), are not divided into
parasympathetic and sympathetic fibers as the efferent fibers are. Instead, autonomic sensory information is conducted by general visceral afferent fibers.
General visceral afferent sensations are mostly unconscious
visceral motor reflex sensations from hollow organs and glands that are
transmitted to the CNS. While the unconscious reflex arcs normally are undetectable, in certain instances they may send pain sensations to the CNS masked as referred pain. If the peritoneal cavity becomes inflamed or if the bowel is suddenly distended, the body will interpret the afferent pain stimulus as somatic in origin. This pain is usually non-localized. The pain is also usually referred to dermatomes that are at the same spinal nerve level as the visceral afferent synapse.
Relationship with the parasympathetic nervous system
Together with the other component of the autonomic nervous system,
the parasympathetic nervous system, the sympathetic nervous system aids
in the control of most of the body's internal organs. Reaction to stress—as in the flight-or-fight response—is thought to counteract the parasympathetic system,
which generally works to promote maintenance of the body at rest. The
comprehensive functions of both the parasympathetic and sympathetic
nervous systems are not so straightforward, but this is a useful rule of
thumb.
Disorders
In heart failure,
the sympathetic nervous system increases its activity, leading to
increased force of muscular contractions that in turn increases the stroke volume, as well as peripheral vasoconstriction to maintain blood pressure. However, these effects accelerate disease progression, eventually increasing mortality in heart failure.
Sympathicotonia is a stimulated condition of the sympathetic nervous system, marked by vascular spasm, elevated blood pressure, and goose bumps.
A recent study has shown the expansion of Foxp3+ natural Treg in the
bone marrow of mice after brain ischemia and this myeloid Treg expansion
is related to sympathetic stress signaling after brain ischemia.
History and etymology
The name of this system can be traced to the concept of sympathy, in the sense of "connection between parts", first used medically by Galen. In the 18th century, Jacob B. Winslow applied the term specifically to nerves.