Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electrons are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin-orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinides, spin-orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins.
Magnetic susceptibility
The primary measurement in magnetochemistry is magnetic
susceptibility. This measures the strength of interaction on placing the
substance in a magnetic field. The volume magnetic susceptibility, represented by the symbol is defined by the relationship
where, is the magnetization of the material (the magnetic dipole moment per unit volume), measured in amperes per meter ( SI units), and is the magnetic field strength, also measured in amperes per meter. Susceptibility is a dimensionless quantity. For chemical applications the molar magnetic susceptibility (χmol) is the preferred quantity. It is measured in m3·mol−1 (SI) or cm3·mol−1 (CGS) and is defined as
where ρ is the density in kg·m−3 (SI) or g·cm−3 (CGS) and M is molar mass in kg·mol−1 (SI) or g·mol−1 (CGS).
Schematic diagram of Gouy balance
A variety of methods are available for the measurement of magnetic susceptibility.
- With the Gouy balance the weight change of the sample is measured with an analytical balance when the sample is placed in a homogeneous magnetic field. The measurements are calibrated against a known standard, such as mercury cobalt thiocyanate, HgCo(NCS)4. Calibration removes the need to know the density of the sample. Variable temperature measurements can be made by placing the sample in a cryostat between the pole pieces of the magnet.
- The Evans balance is a torsion balance which uses a sample in a fixed position and a variable secondary magnet to bring the magnets back to their initial position. It, too, is calibrated against HgCo(NCS)4.
- With a Faraday balance the sample is placed in a magnetic field of constant gradient, and weighed on a torsion balance. This method can yield information on magnetic anisotropy.
- SQUID is a very sensitive magnetometer.
- For substances in solution NMR may be used to measure susceptibility.
Types of magnetic behaviour
When an isolated atom is placed in a magnetic field there is an interaction because each electron in the atom behaves like a magnet, that is, the electron has a magnetic moment. There are two types of interaction.
- Diamagnetism. When placed in a magnetic field the atom becomes magnetically polarized, that is, it develops an induced magnetic moment. The force of the interaction tends to push the atom out of the magnetic field. By convention diamagnetic susceptibility is given a negative sign. Very frequently diamagnetic atoms have no unpaired electrons ie each electron is paired with another electron in the same atomic orbital. The moments of the two electrons cancel each other out, so the atom has no net magnetic moment. However, for the ion Eu3+ which has six unpaired electrons, the orbital angular momentum cancels out the electron angular momentum, and this ion is diamagnetic at zero Kelvin.
- Paramagnetism. At least one electron is not paired with another. The atom has a permanent magnetic moment. When placed into a magnetic field, the atom is attracted into the field. By convention paramagnetic susceptibility is given a positive sign.
When the atom is present in a chemical compound
its magnetic behaviour is modified by its chemical environment.
Measurement of the magnetic moment can give useful chemical information.
In certain crystalline materials individual magnetic moments may
be aligned with each other (magnetic moment has both magnitude and
direction). This gives rise to ferromagnetism, antiferromagnetism or ferrimagnetism. These are properties of the crystal as a whole, of little bearing on chemical properties.
Diamagnetism
Diamagnetism is a universal property of chemical compounds, because
all chemical compounds contain electron pairs. A compound in which there
are no unpaired electrons is said to be diamagnetic. The effect is weak
because it depends on the magnitude of the induced magnetic moment. It
depends on the number of electron pairs and the chemical nature of the
atoms to which they belong. This means that the effects are additive,
and a table of "diamagnetic contributions", or Pascal's constants, can be put together.
With paramagnetic compounds the observed susceptibility can be adjusted
by adding to it the so-called diamagnetic correction, which is the
diamagnetic susceptibility calculated with the values from the table.
Paramagnetism
Mechanism and temperature dependence
Variation of magnetic susceptibility with temperature
A metal ion with a single unpaired electron, such as Cu2+,
in a coordination complex provides the simplest illustration of the
mechanism of paramagnetism. The individual metal ions are kept far apart
by the ligands, so that there is no magnetic interaction between them.
The system is said to be magnetically dilute. The magnetic dipoles of
the atoms point in random directions. When a magnetic field is applied,
first-order Zeeman splitting
occurs. Atoms with spins aligned to the field slightly outnumber the
atoms with non-aligned spins. In the first-order Zeeman effect the
energy difference between the two states is proportional to the applied
field strength. Denoting the energy difference as ΔE, the Boltzmann distribution gives the ratio of the two populations as , where k is the Boltzmann constant and T is the temperature in kelvins. In most cases ΔE is much smaller than kT and the exponential can be expanded as 1 – ΔE/kT. It follows from the presence of 1/T in this expression that the susceptibility is inversely proportional to temperature.
This is known as the Curie law and the proportionality constant, C, is known as the Curie constant, whose value, for molar susceptibility, is calculated as
where N is the Avogadro constant, g is the Landé g-factor, and μB is the Bohr magneton. In this treatment it has been assumed that the electronic ground state
is not degenerate, that the magnetic susceptibility is due only to
electron spin and that only the ground state is thermally populated.
While some substances obey the Curie law, others obey the Curie-Weiss law.
Tc is the Curie temperature.
The Curie-Weiss law will apply only when the temperature is well above
the Curie temperature. At temperatures below the Curie temperature the
substance may become ferromagnetic. More complicated behaviour is observed with the heavier transition elements.
Effective magnetic moment
When the Curie law is obeyed, the product of molar susceptibility and temperature is a constant. The effective magnetic moment, μeff is then defined as
Where C has CGS units cm3 mol−1 K, μeff is
Where C has SI units m3 mol−1 K, μeff is
The quantity μeff is effectively dimensionless, but is often stated as in units of Bohr magneton (μB).
For substances that obey the Curie law, the effective magnetic moment is independent of temperature. For other substances μeff is temperature dependent, but the dependence is small if the Curie-Weiss law holds and the Curie temperature is low.
Temperature independent paramagnetism
Compounds
which are expected to be diamagnetic may exhibit this kind of weak
paramagnetism. It arises from a second-order Zeeman effect in which
additional splitting, proportional to the square of the field strength,
occurs. It is difficult to observe as the compound inevitably also
interacts with the magnetic field in the diamagnetic sense.
Nevertheless, data are available for the permanganate ion. It is easier to observe in compounds of the heavier elements, such as uranyl compounds.
Exchange interactions
Copper(II) acetate dihydrate
Ferrimagnetic ordering in 2 dimensions
Antiferromagnetic ordering in 2 dimensions
Exchange interactions occur when the substance is not magnetically
dilute and there are interactions between individual magnetic centres.
One of the simplest systems to exhibit the result of exchange
interactions is crystalline copper(II) acetate, Cu2(OAc)4(H2O)2. As the formula indicates, it contains two copper(II) ions. The Cu2+ ions are held together by four acetate ligands, each of which binds to both copper ions. Each Cu2+ ion has a d9
electronic configuration, and so should have one unpaired electron. If
there were a covalent bond between the copper ions, the electrons would
pair up and the compound would be diamagnetic. Instead, there is an
exchange interaction in which the spins of the unpaired electrons become
partially aligned to each other. In fact two states are created, one
with spins parallel and the other with spins opposed. The energy
difference between the two states is so small their populations vary
significantly with temperature. In consequence the magnetic moment
varies with temperature in a sigmoidal pattern. The state with spins opposed has lower energy, so the interaction can be classed as antiferromagnetic in this case. It is believed that this is an example of superexchange, mediated by the oxygen and carbon atoms of the acetate ligands. Other dimers and clusters exhibit exchange behaviour.
Exchange interactions can act over infinite chains in one
dimension, planes in two dimensions or over a whole crystal in three
dimensions. These are examples of long-range magnetic ordering. They
give rise to ferromagnetism, antiferromagnetism or ferrimagnetism, depending on the nature and relative orientations of the individual spins.
Compounds at temperatures below the Curie temperature exhibit
long-range magnetic order in the form of ferromagnetism. Another
critical temperature is the Néel temperature, below which antiferromagnetism occurs. The hexahydrate of nickel chloride, NiCl2·6H2O,
has a Néel temperature of 8.3 K. The susceptibility is a maximum at
this temperature. Below the Néel temperature the susceptibility
decreases and the substance becomes antiferromagnetic.
Complexes of transition metal ions
The
effective magnetic moment for a compound containing a transition metal
ion with one or more unpaired electrons depends on the total orbital and
spin angular momentum of the unpaired electrons, and , respectively. "Total" in this context means "vector sum". In the approximation that the electronic states of the metal ions are determined by Russell-Saunders coupling and that spin-orbit coupling is negligible, the magnetic moment is given by
Spin-only formula
Orbital
angular momentum is generated when an electron in an orbital of a
degenerate set of orbitals is moved to another orbital in the set by
rotation. In complexes of low symmetry certain rotations are not possible. In that case the orbital angular momentum is said to be "quenched" and
is smaller than might be expected (partial quenching), or zero
(complete quenching). There is complete quenching in the following
cases. Note that an electron in a degenerate pair of dx2–y2 or dz2 orbitals cannot rotate into the other orbital because of symmetry.
-
Quenched orbital angular momentum dn Octahedral Tetrahedral high-spin low-spin d1 e1 d2 e2 d3 t2g3 d4 t2g3eg1 d5 t2g3eg2 d6 t2g6 e3t23 d7 t2g6eg1 e4t23 d8 t2g6eg2 d9 t2g6eg3
- legend: t2g, t2 = (dxy, dxz, dyz). eg, e = (dx2–y2, dz2).
When orbital angular momentum is completely quenched,
and the paramagnetism can be attributed to electron spin alone. The
total spin angular momentum is simply half the number of unpaired
electrons and the spin-only formula results.
where n is the number of unpaired electrons. The spin-only formula is a good first approximation for high-spin complexes of first-row transition metals.
Ion Number of
unpaired
electronsSpin-only
moment /μBobserved
moment /μBTi3+ 1 1.73 1.73 V4+ 1 1.68–1.78 Cu2+ 1 1.70–2.20 V3+ 2 2.83 2.75–2.85 Ni2+ 2 2.8–3.5 V2+ 3 3.87 3.80–3.90 Cr3+ 3 3.70–3.90 Co2+ 3 4.3–5.0 Mn4+ 3 3.80–4.0 Cr2+ 4 4.90 4.75–4.90 Fe2+ 4 5.1–5.7 Mn2+ 5 5.92 5.65–6.10 Fe3+ 5 5.7–6.0
The small deviations from the spin-only formula may result from the
neglect of orbital angular momentum or of spin-orbit coupling. For
example, tetrahedral d3, d4, d8 and d9
complexes tend to show larger deviations from the spin-only formula
than octahedral complexes of the same ion, because "quenching" of the
orbital contribution is less effective in the tetrahedral case.
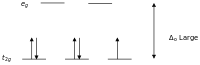
Low-spin complexes
Crystal field diagram for octahedral low-spin d5
Crystal field diagram for octahedral high-spin d5
According to crystal field theory, the d orbitals of a
transition metal ion in an octahedal complex are split into two groups
in a crystal field. If the splitting is large enough to overcome the
energy needed to place electrons in the same orbital, with opposite
spin, a low-spin complex will result.
High and low -spin octahedral complexes d-count Number of unpaired electrons examples high-spin low-spin d4 4 2 Cr2+, Mn3+ d5 5 1 Mn2+, Fe3+ d6 4 0 Fe2+, Co3+ d7 3 1 Co2+
With one unpaired electron μeff values range from 1.8 to 2.5 μB and with two unpaired electrons the range is 3.18 to 3.3 μB. Note that low-spin complexes of Fe2+ and Co3+ are diamagnetic. Another group of complexes that are diamagnetic are square-planar complexes of d8 ions such as Ni2+ and Rh+ and Au3+.
Spin cross-over
When the energy difference between the high-spin and low-spin states is comparable to kT (k is the Boltzmann constant
and T the temperature) an equilibrium is established between the spin
states, involving what have been called "electronic isomers". Tris-dithiocarbamato iron(III), Fe(S2CNR2)3, is a well-documented example. The effective moment varies from a typical d5 low-spin value of 2.25 μB at 80 K to more than 4 μB above 300 K.
2nd and 3rd row transition metals
Crystal
field splitting is larger for complexes of the heavier transition
metals than for the transition metals discussed above. A consequence of
this is that low-spin complexes are much more common. Spin-orbit
coupling constants, ζ, are also larger and cannot be ignored, even in
elementary treatments. The magnetic behaviour has been summarized, as
below, together with an extensive table of data.
d-count kT/ζ=0.1
μeffkT/ζ=0
μeffBehaviour with large spin-orbit coupling constant, ζnd d1 0.63 0 μeff varies with T1/2 d2 1.55 1.22 μeff varies with T, approximately d3 3.88 3.88 Independent of temperature d4 2.64 0 μeff varies with T1/2 d5 1.95 1.73 μeff varies with T, approximately
Lanthanides and actinides
Russell-Saunders coupling,
LS coupling, applies to the lanthanide ions, crystal field effects can
be ignored, but spin-orbit coupling is not negligible. Consequently,
spin and orbital angular momenta have to be combined
and the calculated magnetic moment is given by
| lanthanide | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of unpaired électrons | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| calculated moment /μB | 2.54 | 3.58 | 3.62 | 2.68 | 0.85 | 0 | 7.94 | 9.72 | 10.65 | 10.6 | 9.58 | 7.56 | 4.54 | 0 |
| observed moment /μB | 2.3–2.5 | 3.4–3.6 | 3.5–3.6 | 1.4–1.7 | 3.3–3.5 | 7.9–8.0 | 9.5–9.8 | 10.4–10.6 | 10.4–10.7 | 9.4–9.6 | 7.1–7.5 | 4.3–4.9 | 0 |
In actinides spin-orbit coupling is strong and the coupling approximates to j j coupling.
This means that it is difficult to calculate the effective moment. For example, uranium(IV), f2, in the complex [UCl6]2− has a measured effective moment of 2.2 μB, which includes a contribution from temperature-independent paramagnetism.
Main group elements and organic compounds
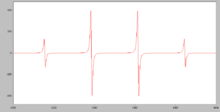
Simulated EPR spectrum of the CH3• radical
MSTL spin-label
Very few compounds of main group elements are paramagnetic. Notable examples include: oxygen, O2; nitric oxide, NO; nitrogen dioxide, NO2 and chlorine dioxide, ClO2. In organic chemistry, compounds with an unpaired electron are said to be free radicals.
Free radicals, with some exceptions, are short-lived because one free
radical will react rapidly with another, so their magnetic properties
are difficult to study. However, if the radicals are well separated from
each other in a dilute solution in a solid matrix, at low temperature,
they can be studied by electron paramagnetic resonance
(EPR). Such radicals are generated by irradiation. Extensive EPR
studies have revealed much about electron delocalization in free
radicals. The simulated spectrum of the CH3• radical shows hyperfine splitting due to the interaction of the electron with the 3 equivalent hydrogen nuclei, each of which has a spin of 1/2.
Spin labels are long-lived free radicals which can be inserted into organic molecules so that they can be studied by EPR. For example, the nitroxide MTSL, a functionalized derivative of TEtra Methyl Piperidine Oxide, TEMPO, is used in site-directed spin labeling.
Applications
The gadolinium ion, Gd3+, has the f7 electronic configuration, with all spins parallel. Compounds of the Gd3+ ion are the most suitable for use as a contrast agent for MRI scans. The magnetic moments of gadolinium compounds are larger than those of any transition metal ion. Gadolinium is
preferred to other lanthanide ions, some of which have larger effective moments, due to its having a non-degenerate electronic ground state.
For many years the nature of oxyhemoglobin, Hb-O2,
was highly controversial. It was found experimentally to be
diamagnetic. Deoxy-hemoglobin is generally accepted to be a complex of
iron in the +2 oxidation state, that is a d6 system with a high-spin magnetic moment near to the spin-only value of 4.9 μB. It was proposed that the iron is oxidized and the oxygen reduced to superoxide.
- Fe(II)Hb (high-spin) + O2 ⇌ [Fe(III)Hb]O2−
Pairing up of electrons from Fe3+ and O2−
was then proposed to occur via an exchange mechanism. It has now been
shown that in fact the iron(II) changes from high-spin to low-spin when
an oxygen molecule donates a pair of electrons to the iron. Whereas in
deoxy-hemoglobin the iron atom lies above the plane of the heme, in the
low-spin complex the effective ionic radius is reduced and the iron atom lies in the heme plane.
- Fe(II)Hb + O2 ⇌ [Fe(II)Hb]O2 (low-spin)
This information has an important bearing on research to find artificial oxygen carriers.
Compounds of gallium(II) were unknown until quite recently. As the atomic number of gallium is an odd number (31), Ga2+ should have an unpaired electron. It was assumed that it would act as a free radical and have a very short lifetime. The non-existence of Ga(II) compounds was part of the so-called inert pair effect. When salts of the anion with empirical formula such as [GaCl3]− were synthesized they were found to be diamagnetic. This implied the formation of a Ga-Ga bond and a dimeric formula, [Ga2Cl6]2−.