From Wikipedia, the free encyclopedia
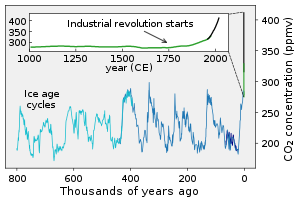
CO2 concentrations over the last 800,000 years
Carbon dioxide (CO2) is an important trace gas in Earth's atmosphere. It is an integral part of the carbon cycle, a biogeochemical cycle in which carbon is exchanged between the Earth's oceans, soil, rocks and the biosphere. Plants and other photoautotrophs use solar energy to produce carbohydrate from atmospheric carbon dioxide and water by photosynthesis.
Almost all other organisms depend on carbohydrate derived from
photosynthesis as their primary source of energy and carbon compounds.
CO2 absorbs and emits infrared radiation at wavelengths of 4.26 μm (2347 cm−1) (asymmetric stretching vibrational mode) and 14.99 μm (667 cm−1) (bending vibrational mode) and consequently is a greenhouse gas that plays a significant role in influencing Earth's surface temperature through the greenhouse effect.
Concentrations of CO2 in the atmosphere were as high as 4,000 parts per million (ppm, on a molar basis) during the Cambrian period about 500 million years ago to as low as 180 ppm during the Quaternary glaciation of the last two million years. Reconstructed temperature records for the last 420 million years indicate that atmospheric CO2 concentrations peaked at ~2000 ppm during the Devonian (~400 Myrs ago) period, and again in the Triassic (220–200 Myrs ago) period. Global annual mean CO2 concentration has increased by 50% since the start of the Industrial Revolution, from 280 ppm during the 10,000 years up to the mid-18th century to 421 ppm as of May 2022. The present concentration is the highest for 14 million years. The increase has been attributed to human activity, particularly deforestation and the burning of fossil fuels. This increase of CO2 and other long-lived greenhouse gases in Earth's atmosphere has produced the current episode of global warming. Between 30% and 40% of the CO2 released by humans into the atmosphere dissolves into the oceans, wherein it forms carbonic acid and effects changes in the oceanic pH balance.
Between 1850 and 2019 the
Global Carbon Project
estimates that about 2/3rds of excess carbon dioxide emissions have
been caused by burning fossil fuels, and a little less than half of that
has stayed in the atmosphere.
Current concentration
Carbon dioxide concentrations have shown several cycles of variation
from about 180 parts per million during the deep glaciations of the Holocene and Pleistocene to 280 parts per million during the interglacial periods. Following the start of the Industrial Revolution, atmospheric CO2 concentration increased to over 400 parts per million and continues to increase, causing the phenomenon of global warming. As of May 2022, the average monthly level of CO2 in Earth's atmosphere reached 421 parts per million. The daily average concentration of atmospheric CO2 at Mauna Loa Observatory first exceeded 400 ppm on 10 May 2013 although this concentration had already been reached in the Arctic in June 2012. Each part per million by volume of CO2 in the atmosphere represents approximately 2.13 gigatonnes of carbon, or 7.82 gigatonnes of CO2. As of 2018, CO2 constitutes about 0.041% by volume of the atmosphere, (equal to 410 ppm) which corresponds to approximately 3210 gigatonnes of CO2, containing approximately 875 gigatonnes of carbon. The global mean CO2 concentration is currently rising at a rate of approximately 2 ppm/year and accelerating. The current growth rate at Mauna Loa is 2.50 ± 0.26 ppm/year (mean ± 2 std dev).
As seen in the graph to the right, there is an annual fluctuation – the
level drops by about 6 or 7 ppm (about 50 Gt) from May to September
during the Northern Hemisphere's growing season, and then goes up by about 8 or 9 ppm. The Northern Hemisphere dominates the annual cycle of CO2 concentration because it has much greater land area and plant biomass than the Southern Hemisphere.
Concentrations reach a peak in May as the Northern Hemisphere spring
greenup begins, and decline to a minimum in October, near the end of the
growing season.
Since global warming is attributed to increasing atmospheric concentrations of greenhouse gases such as CO2 and methane, scientists closely monitor atmospheric CO2 concentrations and their impact on the present-day biosphere. The National Geographic
wrote that the concentration of carbon dioxide in the atmosphere is
this high "for the first time in 55 years of measurement—and probably
more than 3 million years of Earth history." The current concentration may be the highest in the last 20 million years.
Past concentration
Concentration of atmospheric CO
2 over the last 40,000 years, from the
Last Glacial Maximum to the present day. The current rate of increase is much higher than at any point during the last
deglaciation.
Carbon dioxide concentrations have varied widely over the Earth's
4.54 billion year history. It is believed to have been present in
Earth's first atmosphere, shortly after Earth's formation. The second
atmosphere, consisting largely of nitrogen and CO
2 was produced by outgassing from volcanism, supplemented by gases produced during the late heavy bombardment of Earth by huge asteroids. A major part of carbon dioxide emissions were soon dissolved in water and incorporated in carbonate sediments.
The production of free oxygen by cyanobacterial photosynthesis eventually led to the oxygen catastrophe
that ended Earth's second atmosphere and brought about the Earth's
third atmosphere (the modern atmosphere) 2.4 billion years before the
present. Carbon dioxide concentrations dropped from 4,000 parts per
million during the Cambrian period about 500 million years ago to as low as 180 parts per million during the Quaternary glaciation of the last two million years.
Drivers of ancient-Earth CO2 concentration
On long timescales, atmospheric CO2 concentration is determined by the balance among geochemical processes including organic carbon burial in sediments, silicate rock weathering, and volcanic degassing. The net effect of slight imbalances in the carbon cycle over tens to hundreds of millions of years has been to reduce atmospheric CO2.
On a timescale of billions of years, such downward trend appears bound
to continue indefinitely as occasional massive historical releases of
buried carbon due to volcanism will become less frequent (as earth
mantle cooling and progressive exhaustion of internal radioactive heat proceed further). The rates of these processes are extremely slow; hence they are of no relevance to the atmospheric CO2 concentration over the next hundreds or thousands of years.
In billion-year timescales, it is predicted
that plant, and therefore animal, life on land will die off altogether,
since by that time most of the remaining carbon in the atmosphere will
be sequestered underground, and natural releases of CO2 by radioactivity-driven tectonic activity will have continued to slow down.
The loss of plant life would also result in the eventual loss of
oxygen. Some microbes are capable of photosynthesis at concentrations of
CO2 of a few
parts per million and so the last life forms would probably disappear
finally due to the rising temperatures and loss of the atmosphere when
the sun becomes a red giant some four billion years from now.
Measuring ancient-Earth CO2 concentration
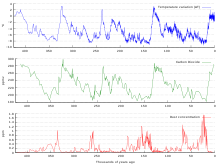
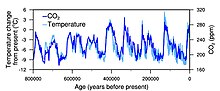
Graph of CO2 (green), reconstructed temperature (blue) and dust (red) from the Vostok ice core for the past 420,000 years
Correspondence between temperature and atmospheric CO2 during the last 800,000 years
The most direct method for measuring atmospheric carbon dioxide
concentrations for periods before instrumental sampling is to measure
bubbles of air (fluid or gas inclusions) trapped in the Antarctic or Greenland ice sheets. The most widely accepted of such studies come from a variety of Antarctic cores and indicate that atmospheric CO2
concentrations were about 260–280 ppmv immediately before industrial
emissions began and did not vary much from this level during the
preceding 10,000 years. The longest ice core record comes from East Antarctica, where ice has been sampled to an age of 800,000 years. During this time, the atmospheric carbon dioxide concentration has varied between 180 and 210 ppm during ice ages, increasing to 280–300 ppm during warmer interglacials. The beginning of human agriculture during the current Holocene epoch may have been strongly connected to the atmospheric CO2 increase after the last ice age ended, a fertilization effect raising plant biomass growth and reducing stomatal conductance requirements for CO2 intake, consequently reducing transpiration water losses and increasing water usage efficiency.
Various proxy measurements have been used to attempt to determine atmospheric carbon dioxide concentrations millions of years in the past. These include boron and carbon isotope ratios in certain types of marine sediments, and the number of stomata observed on fossil plant leaves.
Phytane is a type of diterpenoid alkane. It is a breakdown product of chlorophyll and is now used to estimate ancient CO2 levels. Phytane gives both a continuous record of CO2 concentrations but it also can overlap a break in the CO2 record of over 500 million years.
400 to 600 M yrs ago
There is evidence for high CO2
concentrations between 200 and 150 million years ago of over 3,000 ppm,
and between 600 and 400 million years ago of over 6,000 ppm.
5 to 60 M yrs ago
In more recent times, atmospheric CO2 concentration continued to fall after about 60 million years ago. About 34 million years ago, the time of the Eocene–Oligocene extinction event and when the Antarctic ice sheet started to take its current form, CO2 was about 760 ppm, and there is geochemical evidence that concentrations were less than 300 ppm by about 20 million years ago. Decreasing CO2 concentration, with a tipping point of 600 ppm, was the primary agent forcing Antarctic glaciation. Low CO2 concentrations may have been the stimulus that favored the evolution of C4 plants, which increased greatly in abundance between 7 and 5 million years ago.
CO2 possibly higher 7,000 - 10,000 yrs ago
Based on an analysis of fossil leaves, Wagner et al. argued that atmospheric CO2
concentrations during the last 7,000–10,000 year period were
significantly higher than 300 ppm and contained substantial variations
that may be correlated to climate variations. Others have disputed such
claims, suggesting they are more likely to reflect calibration problems
than actual changes in CO2. Relevant to this dispute is the observation that Greenland ice cores often report higher and more variable CO2 values than similar measurements in Antarctica. However, the groups responsible for such measurements (e.g. H.J. Smith et al.) believe the variations in Greenland cores result from in situ decomposition of calcium carbonate
dust found in the ice. When dust concentrations in Greenland cores are
low, as they nearly always are in Antarctic cores, the researchers
report good agreement between measurements of Antarctic and Greenland CO2 concentrations.
Atmospheric CO2 and the greenhouse effect
A pictogram of the greenhouse effect
Earth's natural greenhouse effect
makes life as we know it possible and carbon dioxide plays a
significant role in providing for the relatively high temperature that
the planet enjoys. The greenhouse effect is a process by which thermal
radiation from a planetary atmosphere warms the planet's surface beyond
the temperature it would have in the absence of its atmosphere. Without the greenhouse effect, the Earth's average surface temperature would be about −18 °C (−0.4 °F) compared to Earth's actual average surface temperature of approximately 14 °C (57.2 °F).
Carbon dioxide is believed to have played an important effect in
regulating Earth's temperature throughout its 4.7 billion year history.
Early in the Earth's life, scientists have found evidence of liquid
water indicating a warm world even though the Sun's output is believed
to have only been 70% of what it is today. It has been suggested by
scientists that higher carbon dioxide concentrations in the early
Earth's atmosphere might help explain this faint young sun paradox. When Earth first formed, Earth's atmosphere may have contained more greenhouse gases and CO2 concentrations may have been higher, with estimated partial pressure as large as 1,000 kPa (10 bar), because there was no bacterial photosynthesis to reduce the gas to carbon compounds and oxygen. Methane, a very active greenhouse gas which reacts with oxygen to produce CO2 and water vapor, may have been more prevalent as well, with a mixing ratio of 10−4 (100 parts per million by volume).
Radiative forcing drivers of climate change in year 2011, relative to pre-industrial (1750).
Though water is responsible for most (about 36-70%) of the total greenhouse effect, the role of water vapor
as a greenhouse gas depends on temperature. On Earth, carbon dioxide is
the most relevant, direct anthropologically influenced greenhouse gas.
Carbon dioxide is often mentioned in the context of its increased
influence as a greenhouse gas since the pre-industrial (1750) era. In
the IPCC Fifth Assessment Report the increase in CO2 was estimated to be responsible for 1.82 W m−2 of the 2.63 W m−2 change in radiative forcing on Earth (about 70%).
The concept of atmospheric CO2 increasing ground temperature was first published by Svante Arrhenius in 1896. The increased radiative forcing due to increased CO2 in the Earth's atmosphere is based on the physical properties of CO2 and the non-saturated absorption windows where CO2 absorbs outgoing long-wave energy. The increased forcing drives further changes in Earth's energy balance and, over the longer term, in Earth's climate.
Atmospheric CO2 and the carbon cycle
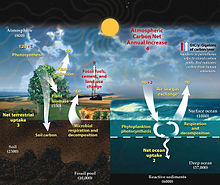
This
diagram of the fast carbon cycle shows the movement of carbon between
land, atmosphere, and oceans in billions of metric tons of carbon per
year. Yellow numbers are natural fluxes, red are human contributions,
white are stored carbon.
Atmospheric carbon dioxide plays an integral role in the Earth's carbon cycle whereby CO2 is removed from the atmosphere by some natural processes such as photosynthesis
and deposition of carbonates, to form limestones for example, and added
back to the atmosphere by other natural processes such as respiration
and the acid dissolution of carbonate deposits. There are two broad
carbon cycles on Earth: the fast carbon cycle and the slow carbon cycle.
The fast carbon cycle refers to movements of carbon between the
environment and living things in the biosphere whereas the slow carbon
cycle involves the movement of carbon between the atmosphere, oceans,
soil, rocks, and volcanism. Both cycles are intrinsically interconnected
and atmospheric CO2 facilitates the linkage.
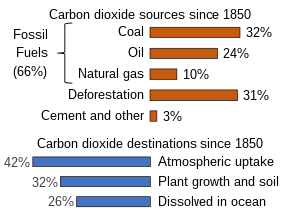
Natural sources of atmospheric CO2 include volcanic outgassing, the combustion of organic matter, wildfires and the respiration processes of living aerobic organisms. Man-made sources of CO2 include the burning of fossil fuels for heating, power generation and transport, as well as some industrial processes such as cement making. It is also produced by various microorganisms from fermentation and cellular respiration. Plants, algae and cyanobacteria convert carbon dioxide to carbohydrates by a process called photosynthesis. They gain the energy needed for this reaction from absorption of sunlight by chlorophyll
and other pigments. Oxygen, produced as a by-product of photosynthesis,
is released into the atmosphere and subsequently used for respiration
by heterotrophic organisms and other plants, forming a cycle with carbon.
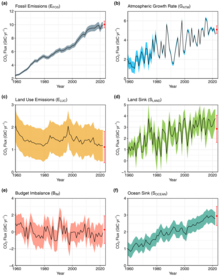
Annual CO2
flows from anthropogenic sources (left) into Earth's atmosphere, land,
and ocean sinks (right) since year 1960. Units in equivalent gigatonnes
carbon per year.
Most sources of CO2 emissions are natural, and are balanced to various degrees by similar CO2
sinks. For example, the decay of organic material in forests,
grasslands, and other land vegetation - including forest fires - results
in the release of about 436 gigatonnes of CO2 (containing 119 gigatonnes carbon) every year, while CO2 uptake by new growth on land counteracts these releases, absorbing 451 Gt (123 Gt C). Although much CO2 in the early atmosphere of the young Earth was produced by volcanic activity, modern volcanic activity releases only 130 to 230 megatonnes of CO2 each year. Natural sources are more or less balanced by natural sinks, in the form of chemical and biological processes which remove CO2
from the atmosphere. By contrast, as of year 2019 the extraction and
burning of geologic fossil carbon by humans releases over 30 gigatonnes
of CO2 (9 billion tonnes carbon) each year. This larger disruption to the natural balance is responsible for recent growth in the atmospheric CO2 concentration.
Overall, there is a large natural flux of atmospheric CO2 into and out of the biosphere, both on land and in the oceans. In the pre-industrial era, each of these fluxes were in balance to such a degree that little net CO2
flowed between the land and ocean reservoirs of carbon, and little
change resulted in the atmospheric concentration. From the human
pre-industrial era to 1940, the terrestrial biosphere represented a net
source of atmospheric CO2 (driven largely by land-use changes), but subsequently switched to a net sink with growing fossil carbon emissions. In 2012, about 57% of human-emitted CO2, mostly from the burning of fossil carbon, was taken up by land and ocean sinks.
The ratio of the increase in atmospheric CO2 to emitted CO2 is known as the airborne fraction (Keeling et al., 1995). This ratio varies in the short-term and is typically about 45% over longer (5-year) periods.
Estimated carbon in global terrestrial vegetation increased from
approximately 740 gigatonnes in 1910 to 780 gigatonnes in 1990. By 2009, oceanic neutralization had decreased the pH of seawater by 0.11 due to uptake of emitted CO2.
Atmospheric CO2 and photosynthesis
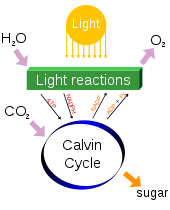
Photosynthesis changes sunlight into chemical energy, splits water to liberate O2, and fixes CO2 into sugar.
Carbon dioxide in the Earth's atmosphere is essential to life and to
most of the planetary biosphere. Over the course of Earth's geologic
history CO2 concentrations have played a role in biological evolution. The first photosynthetic organisms probably evolved early in the evolutionary history of life and most likely used reducing agents such as hydrogen or hydrogen sulfide as sources of electrons, rather than water. Cyanobacteria appeared later, and the excess oxygen they produced contributed to the oxygen catastrophe, which rendered the evolution of complex life possible. In recent geologic times, low CO2 concentrations below 600 parts per million might have been the stimulus that favored the evolution of C4 plants which increased greatly in abundance between 7 and 5 million years ago over plants that use the less efficient C3 metabolic pathway. At current atmospheric pressures photosynthesis shuts down when atmospheric CO2
concentrations fall below 150 ppm and 200 ppm although some microbes
can extract carbon from the air at much lower concentrations. Today, the average rate of energy capture by photosynthesis globally is approximately 130 terawatts, which is about six times larger than the current power consumption of human civilization. Photosynthetic organisms also convert around 100–115 billion metric tonnes of carbon into biomass per year.
Photosynthetic organisms are photoautotrophs, which means that they are able to synthesize food directly from CO2
and water using energy from light. However, not all organisms that use
light as a source of energy carry out photosynthesis, since photoheterotrophs use organic compounds, rather than CO2, as a source of carbon. In plants, algae and cyanobacteria, photosynthesis releases oxygen. This is called oxygenic photosynthesis. Although there are some differences between oxygenic photosynthesis in plants, algae, and cyanobacteria, the overall process is quite similar in these organisms. However, there are some types of bacteria that carry out anoxygenic photosynthesis, which consumes CO2 but does not release oxygen.
Carbon dioxide is converted into sugars in a process called carbon fixation. Carbon fixation is an endothermic redox
reaction, so photosynthesis needs to supply both the source of energy
to drive this process and the electrons needed to convert CO2 into a carbohydrate. This addition of the electrons is a reduction reaction. In general outline and in effect, photosynthesis is the opposite of cellular respiration, in which glucose and other compounds are oxidized to produce CO2 and water, and to release exothermic chemical energy to drive the organism's metabolism.
However, the two processes take place through a different sequence of
chemical reactions and in different cellular compartments.
Most organisms that utilize photosynthesis to produce oxygen use visible light to do so, although at least three use shortwave infrared or, more specifically, far-red radiation.
Effects of increased CO2 on plants and crops
A 1993 review of scientific greenhouse studies found that a doubling of CO2
concentration would stimulate the growth of 156 different plant species
by an average of 37%. Response varied significantly by species, with
some showing much greater gains and a few showing a loss. For example, a
1979 greenhouse study found that with doubled CO2
concentration the dry weight of 40-day-old cotton plants doubled, but
the dry weight of 30-day-old maize plants increased by only 20%.
In addition to greenhouse studies, field and satellite measurements attempt to understand the effect of increased CO2 in more natural environments. In free-air carbon dioxide enrichment (FACE) experiments plants are grown in field plots and the CO2 concentration of the surrounding air is artificially elevated. These experiments generally use lower CO2
levels than the greenhouse studies. They show lower gains in growth
than greenhouse studies, with the gains depending heavily on the species
under study. A 2005 review of 12 experiments at 475–600 ppm showed an
average gain of 17% in crop yield, with legumes typically showing a greater response than other species and C4 plants generally showing less. The review also stated that the experiments have their own limitations. The studied CO2 levels were lower, and most of the experiments were carried out in temperate regions. Satellite measurements found increasing leaf area index
for 25% to 50% of Earth's vegetated area over the past 35 years (i.e., a
greening of the planet), providing evidence for a positive CO2 fertilization effect.
A 2017 Politico article states that increased CO2 levels may have a negative impact on the nutritional quality of various human food crops, by increasing the levels of carbohydrates, such as glucose, while decreasing the levels of important nutrients such as protein, iron, and zinc. Crops experiencing a decrease in protein include rice, wheat, barley and potatoes.
Atmospheric CO2 and the oceanic carbon cycle
The Earth's oceans contain a large amount of CO2
in the form of bicarbonate and carbonate ions—much more than the amount
in the atmosphere. The bicarbonate is produced in reactions between
rock, water, and carbon dioxide. One example is the dissolution of
calcium carbonate:
- CaCO
3 + CO2 + H
2O ⇌ Ca2+
+ 2 HCO−
3
Reactions like this tend to buffer changes in atmospheric CO2. Since the right side of the reaction produces an acidic compound, adding CO2 on the left side decreases the pH of seawater, a process which has been termed ocean acidification (pH of the ocean becomes more acidic although the pH value remains in the alkaline range). Reactions between CO2
and non-carbonate rocks also add bicarbonate to the seas. This can
later undergo the reverse of the above reaction to form carbonate rocks,
releasing half of the bicarbonate as CO2. Over hundreds of millions of years, this has produced huge quantities of carbonate rocks.
Ultimately, most of the CO2 emitted by human activities will dissolve in the ocean;
however, the rate at which the ocean will take it up in the future is
less certain.
Even if equilibrium is reached, including dissolution of carbonate
minerals, the increased concentration of bicarbonate and decreased or
unchanged concentration of carbonate ion will give rise to a higher
concentration of un-ionized carbonic acid and dissolved CO2. This higher concentration in the seas, along with higher temperatures, would mean a higher equilibrium concentration of CO2 in the air.
Carbon dioxide has unique long-term effects on climate change
that are nearly "irreversible" for a thousand years after emissions stop
(zero further emissions). The greenhouse gases methane and nitrous oxide
do not persist over time in the same way as carbon dioxide. Even if
human carbon dioxide emissions were to completely cease, atmospheric
temperatures are not expected to decrease significantly in the short
term. This is because the air temperature is determined by a balance
between heating, due to greenhouse gases, and cooling due to heat
transfer to the ocean. If emissions were to stop, CO2 levels
and the heating effect would slowly decrease, but simultaneously the
cooling due to heat transfer would diminish (because sea temperatures
would get closer to the air temperature), with the result that the air
temperature would decrease only slowly. Sea temperatures would continue
to rise, causing thermal expansion and some sea level rise. Lowering global temperatures more rapidly would require carbon sequestration or geoengineering.
Carbon moves between the atmosphere, vegetation (dead and alive),
the soil, the surface layer of the ocean, and the deep ocean. A
detailed model has been developed by Fortunat Joos in Bern and colleagues, called the Bern model.
A simpler model based on it gives the fraction of CO2 remaining in the atmosphere as a function of the number of years after it is emitted into the atmosphere:

According to this model, 21.7% of the carbon dioxide released into
the air stays there forever, but of course this is not true if
carbon-containing material is removed from the cycle (and stored) in
ways that are not operative at present (artificial sequestration).
Anthropogenic CO2 emissions
The US, China and Russia have cumulatively contributed the greatest amounts of CO2 since 1850.
While CO2 absorption and release is always happening as a result of natural processes, the recent rise in CO2 levels in the atmosphere is known to be mainly due to human (anthropogenic) activity.
There are four ways human activity, especially fossil fuel burning, is
known to have caused the rapid increase in atmospheric CO2 over the last few centuries:
- Various national statistics accounting for fossil fuel consumption, combined with knowledge of how much atmospheric CO2 is produced per unit of fossil fuel (e.g. liter of gasoline).
- By examining the ratio of various carbon isotopes in the atmosphere. The burning of long-buried fossil fuels releases CO2
containing carbon of different isotopic ratios to those of living
plants, enabling distinction between natural and human-caused
contributions to CO2 concentration.
- Higher atmospheric CO2
concentrations in the Northern Hemisphere, where most of the world's
population lives (and emissions originate from), compared to the
southern hemisphere. This difference has increased as anthropogenic
emissions have increased.
- Atmospheric O2 levels are decreasing in Earth's atmosphere as it reacts with the carbon in fossil fuels to form CO2.
Burning fossil fuels such as coal, petroleum, and natural gas is the leading cause of increased anthropogenic CO2; deforestation is the second major cause. In 2010, 9.14 gigatonnes of carbon (GtC, equivalent to 33.5 gigatonnes of CO2
or about 4.3 ppm in Earth's atmosphere) were released from fossil fuels
and cement production worldwide, compared to 6.15 GtC in 1990. In addition, land use change contributed 0.87 GtC in 2010, compared to 1.45 GtC in 1990. In 1997, human-caused Indonesian peat fires were estimated to have released between 13% and 40% of the average annual global carbon emissions caused by the burning of fossil fuels. In the period 1751 to 1900, about 12 GtC were released as CO2 to the atmosphere from burning of fossil fuels, whereas from 1901 to 2013 the figure was about 380 GtC.
The Integrated Carbon Observation System (ICOS) continuously releases data about CO2 emissions, budget and concentration at individual observation stations.
CO2 emissions
| Year
|
Fossil fuels
and industry
Gt C
|
Land use
change
Gt C
|
Total
Gt C
|
Total
Gt CO2
|
| 2010
|
9.05
|
1.38
|
10.43
|
38.2
|
| 2011
|
9.35
|
1.34
|
10.69
|
39.2
|
| 2012
|
9.5
|
1.47
|
10.97
|
40.3
|
| 2013
|
9.54
|
1.52
|
11.06
|
40.6
|
| 2014
|
9.61
|
1.66
|
11.27
|
41.4
|
| 2015
|
9.62
|
1.7
|
11.32
|
41.5
|
| 2016
|
9.66
|
1.54
|
11.2
|
41.1
|
| 2017
|
9.77
|
1.47
|
11.24
|
41.3
|
| 2018
|
9.98
|
1.51
|
11.49
|
42.1
|
2019
(projection)
|
10.0
|
1.8
|
11.8
|
43.1
|
Anthropogenic carbon emissions exceed the amount that can be taken up or balanced out by natural sinks.
As a result, carbon dioxide has gradually accumulated in the
atmosphere, and as of May 2022, its concentration is 50% above
pre-industrial levels. Various techniques have been proposed for removing excess carbon dioxide from the atmosphere (see Carbon sink#Artificial sequestration). Currently about half of the carbon dioxide released from the burning of fossil fuels is not absorbed by vegetation and the oceans and remains in the atmosphere.
Global fossil carbon emissions 1800–2014
False-color image of smoke and ozone pollution from Indonesian fires, 1997
Biosphere CO2 flux in the northern hemisphere winter (NOAA Carbon Tracker)
Biosphere CO2 flux in the northern hemisphere summer (NOAA Carbon Tracker)
Ongoing measurements of atmospheric CO2
Carbon
Dioxide observations from 2005 to 2014 showing the seasonal variations
and the difference between northern and southern hemispheres
The first reproducibly accurate measurements of atmospheric CO2 were from flask sample measurements made by Dave Keeling at Caltech in the 1950s. A few years later in March 1958 the first ongoing measurements were started by Keeling at Mauna Loa.
Measurements at Mauna Loa have been ongoing since then. Now
measurements are made at many sites globally. Additional measurement
techniques are also used as well. Many measurement sites are part of
larger global networks. Global network data are often made publicly
available on the conditions of proper acknowledgment according to the
respective data user policies.
There are several surface measurement (including flasks and continuous in situ) networks including NOAA/ERSL, WDCGG, and RAMCES. The NOAA/ESRL Baseline Observatory Network, and the Scripps Institution of Oceanography Network data are hosted at the CDIAC at ORNL. The World Data Centre for Greenhouse Gases (WDCGG), part of GAW, data are hosted by the JMA. The Reseau Atmospherique de Mesure des Composes an Effet de Serre database (RAMCES) is part of IPSL.
From these measurements, further products are made which
integrate data from the various sources. These products also address
issues such as data discontinuity and sparseness. GLOBALVIEW-CO2 is one
of these products.
Ongoing ground-based total column measurements began more
recently. Column measurements typically refer to an averaged column
amount denoted XCO2, rather than a surface only measurement. These measurements are made by the TCCON. These data are also hosted on the CDIAC, and made publicly available according to the data use policy.
Satellite measurements are also a recent addition to atmospheric XCO2 measurements. SCIAMACHY aboard ESA's ENVISAT made global column XCO2 measurements from 2002 to 2012. AIRS aboard NASA's Aqua satellite makes global XCO2
measurements and was launched shortly after ENVISAT in 2012. More
recent satellites have significantly improved the data density and
precision of global measurements. Newer missions have higher spectral
and spatial resolutions. JAXA's GOSAT was the first dedicated GHG monitoring satellite to successfully achieve orbit in 2009. NASA's OCO-2 launched in 2014 was the second. Various other satellites missions to measure atmospheric XCO2 are planned.