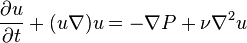From Wikipedia, the free encyclopedia
The lapse rate is defined as the rate at which atmospheric temperature decreases with an increase in altitude.[1][2] The terminology arises from the word lapse in the sense of a decrease or decline. While most often applied to Earth's troposphere, the concept can be extended to any gravitationally supported parcel of gas.
Although the actual atmospheric lapse rate varies, under normal atmospheric conditions the average atmospheric lapse rate results in a temperature decrease of 6.4 °C/km (3.5 °F or 1.95 °C/1,000 ft) of altitude above ground level.
The measurable lapse rate is affected by the moisture content of the air (humidity). A dry lapse rate of 10 °C/km (5.5 °F or 3.05 °C/1,000 ft) is often used to calculate temperature changes in air not at 100% relative humidity. A wet lapse rate of 5.5 °C/km (3 °F or 1.68 °C/1,000 ft) is used to calculate the temperature changes in air that is saturated (i.e., air at 100% relative humidity). Although actual lapse rates do not strictly follow these guidelines, they present a model sufficiently accurate to predict temperate changes associated with updrafts and downdrafts. This differential lapse rate (dependent upon both difference in conductive heating and adiabatic expansion or compression) results in the formation of warm downslope winds (e.g., Chinook winds, Santa Ana winds, etc.). The atmospheric lapse rate, combined with adiabatic cooling and heating of air related to the expansion and compression of atmospheric gases, present a unified model explaining the cooling of air as it moves aloft and the heating of air as it descends downslope.
Atmospheric stability can be measured in terms of lapse rates (i.e., the temperature differences associated with vertical movement of air). The atmosphere is considered conditionally unstable where the environmental lapse rate causes a slower decrease in temperature with altitude than the dry adiabatic lapse rate, as long as no latent heat is released (i.e. the saturated adiabatic lapse rate applies). Unconditional instability results when the dry adiabatic lapse rate causes air to cool slower than the environmental lapse rate, so air will continue to rise until it reaches the same temperature as its surroundings. Where the saturated adiabatic lapse rate is greater than the environmental lapse rate, the air cools faster than its environment and thus returns to its original position, irrespective of its moisture content.
Although the atmospheric lapse rate (also known as the environmental lapse rate) is most often used to characterize temperature changes, many properties (e.g. atmospheric pressure) can also be profiled by lapse rates...
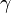 is the lapse rate given in units of temperature divided by units of altitude, T = temperature, and z = altitude.
is the lapse rate given in units of temperature divided by units of altitude, T = temperature, and z = altitude.
Note: In some cases, or
or  can be used to represent the adiabatic lapse rate in order to avoid confusion with other terms symbolized by
can be used to represent the adiabatic lapse rate in order to avoid confusion with other terms symbolized by 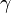 , such as the specific heat ratio[4] or the psychrometric constant.[5]
, such as the specific heat ratio[4] or the psychrometric constant.[5]
The dry adiabatic lapse rate (DALR) is the rate of temperature decrease with altitude for a parcel of dry or unsaturated air rising under adiabatic conditions. Unsaturated air has less than 100% relative humidity; i.e. its actual temperature is higher than its dew point. The term adiabatic means that no heat transfer occurs into or out of the parcel. Air has low thermal conductivity, and the bodies of air involved are very large, so transfer of heat by conduction is negligibly small.
Under these conditions when the air rises (for instance, by convection) it expands, because the pressure is lower at higher altitudes. As the air parcel expands, it pushes on the air around it, doing work (thermodynamics). Since the parcel does work but gains no heat, it loses internal energy so that its temperature decreases. The rate of temperature decrease is 9.8 °C/km (5.38 °F per 1,000 ft) (3.0 °C/1,000 ft). The reverse occurs for a sinking parcel of air.[7]
Since for adiabatic process:
 and :
and : we can show that:
we can show that:
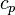 is the specific heat at constant pressure and
is the specific heat at constant pressure and  is the specific volume.
is the specific volume.
Assuming an atmosphere in hydrostatic equilibrium:[8]
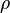 is the density. Combining these two equations to eliminate the pressure, one arrives at the result for the DALR,[9]
is the density. Combining these two equations to eliminate the pressure, one arrives at the result for the DALR,[9]
The reason for the difference between the dry and moist adiabatic lapse rate values is that latent heat is released when water condenses, thus decreasing the rate of temperature drop as altitude increases. This heat release process is an important source of energy in the development of thunderstorms. An unsaturated parcel of air of given temperature, altitude and moisture content below that of the corresponding dew point cools at the dry adiabatic lapse rate as altitude increases until the dew point line for the given moisture content is intersected. As the water vapour then starts condensing the air parcel subsequently cools at the slower moist adiabatic lapse rate if the altitude increases further.
The saturated adiabatic lapse rate is given approximately by this equation from the glossary of the American Meteorology Society:[10]
As unsaturated air rises, its temperature drops at the dry adiabatic rate. The dew point also drops (as a result of decreasing air pressure) but much more slowly, typically about −2 °C per 1,000 m. If unsaturated air rises far enough, eventually its temperature will reach its dew point, and condensation will begin to form. This altitude is known as the lifting condensation level (LCL) when mechanical lift is present and the convective condensation level (CCL) when mechanical lift is absent, in which case, the parcel must be heated from below to its convective temperature. The cloud base will be somewhere within the layer bounded by these parameters.
The difference between the dry adiabatic lapse rate and the rate at which the dew point drops is around 8 °C per 1,000 ft. Given a difference in temperature and dew point readings on the ground, one can easily find the LCL by multiplying the difference by 125 m/°C.
If the environmental lapse rate is less than the moist adiabatic lapse rate, the air is absolutely stable — rising air will cool faster than the surrounding air and lose buoyancy. This often happens in the early morning, when the air near the ground has cooled overnight. Cloud formation in stable air is unlikely.
If the environmental lapse rate is between the moist and dry adiabatic lapse rates, the air is conditionally unstable — an unsaturated parcel of air does not have sufficient buoyancy to rise to the LCL or CCL, and it is stable to weak vertical displacements in either direction. If the parcel is saturated it is unstable and will rise to the LCL or CCL, and either be halted due to an inversion layer of convective inhibition, or if lifting continues, deep, moist convection (DMC) may ensue, as a parcel rises to the level of free convection (LFC), after which it enters the free convective layer (FCL) and usually rises to the equilibrium level (EL).
If the environmental lapse rate is larger than the dry adiabatic lapse rate, it has a superadiabatic lapse rate, the air is absolutely unstable — a parcel of air will gain buoyancy as it rises both below and above the lifting condensation level or convective condensation level. This often happens in the afternoon mainly over land masses. In these conditions, the likelihood of cumulus clouds, showers or even thunderstorms is increased.
Meteorologists use radiosondes to measure the environmental lapse rate and compare it to the predicted adiabatic lapse rate to forecast the likelihood that air will rise. Charts of the environmental lapse rate are known as thermodynamic diagrams, examples of which include Skew-T log-P diagrams and tephigrams.
The difference in moist adiabatic lapse rate and the dry rate is the cause of foehn wind phenomenon (also known as "Chinook winds" in parts of North America).
Definition
A formal definition from the Glossary of Meteorology[3] is:- The decrease of an atmospheric variable with height, the variable being temperature unless otherwise specified.
Although the actual atmospheric lapse rate varies, under normal atmospheric conditions the average atmospheric lapse rate results in a temperature decrease of 6.4 °C/km (3.5 °F or 1.95 °C/1,000 ft) of altitude above ground level.
The measurable lapse rate is affected by the moisture content of the air (humidity). A dry lapse rate of 10 °C/km (5.5 °F or 3.05 °C/1,000 ft) is often used to calculate temperature changes in air not at 100% relative humidity. A wet lapse rate of 5.5 °C/km (3 °F or 1.68 °C/1,000 ft) is used to calculate the temperature changes in air that is saturated (i.e., air at 100% relative humidity). Although actual lapse rates do not strictly follow these guidelines, they present a model sufficiently accurate to predict temperate changes associated with updrafts and downdrafts. This differential lapse rate (dependent upon both difference in conductive heating and adiabatic expansion or compression) results in the formation of warm downslope winds (e.g., Chinook winds, Santa Ana winds, etc.). The atmospheric lapse rate, combined with adiabatic cooling and heating of air related to the expansion and compression of atmospheric gases, present a unified model explaining the cooling of air as it moves aloft and the heating of air as it descends downslope.
Atmospheric stability can be measured in terms of lapse rates (i.e., the temperature differences associated with vertical movement of air). The atmosphere is considered conditionally unstable where the environmental lapse rate causes a slower decrease in temperature with altitude than the dry adiabatic lapse rate, as long as no latent heat is released (i.e. the saturated adiabatic lapse rate applies). Unconditional instability results when the dry adiabatic lapse rate causes air to cool slower than the environmental lapse rate, so air will continue to rise until it reaches the same temperature as its surroundings. Where the saturated adiabatic lapse rate is greater than the environmental lapse rate, the air cools faster than its environment and thus returns to its original position, irrespective of its moisture content.
Although the atmospheric lapse rate (also known as the environmental lapse rate) is most often used to characterize temperature changes, many properties (e.g. atmospheric pressure) can also be profiled by lapse rates...
Mathematical definition
In general, a lapse rate is the negative of the rate of temperature change with altitude change, thus: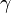 is the lapse rate given in units of temperature divided by units of altitude, T = temperature, and z = altitude.
is the lapse rate given in units of temperature divided by units of altitude, T = temperature, and z = altitude.Note: In some cases,
 or
or  can be used to represent the adiabatic lapse rate in order to avoid confusion with other terms symbolized by
can be used to represent the adiabatic lapse rate in order to avoid confusion with other terms symbolized by 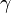 , such as the specific heat ratio[4] or the psychrometric constant.[5]
, such as the specific heat ratio[4] or the psychrometric constant.[5]Types of lapse rates
There are two types of lapse rate:- Environmental lapse rate (ELR) – which refers to the actual change of temperature with altitude for the stationary atmosphere (i.e. the temperature gradient)
- The adiabatic lapse rates – which refer to the change in temperature of a parcel of air as it moves upwards (or downwards) without exchanging heat with its surroundings. There are two adiabatic rates:[6]
- Dry adiabatic lapse rate (DALR)
- Moist (or saturated) adiabatic lapse rate (SALR)
Environmental lapse rate
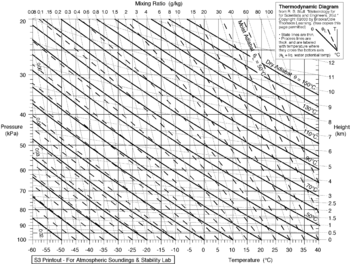
The environmental lapse rate (ELR), is the rate of decrease of temperature with altitude in the stationary atmosphere at a given time and location. As an average, the International Civil Aviation Organization (ICAO) defines an international standard atmosphere (ISA) with a temperature lapse rate of 6.49 K/km[citation needed] (3.56 °F or 1.98 K/1,000 ft) from sea level to 11 km (36,090 ft or 6.8 mi). From 11 km up to 20 km (65,620 ft or 12.4 mi), the constant temperature is −56.5 °C (−69.7 °F), which is the lowest assumed temperature in the ISA. The standard atmosphere contains no moisture. Unlike the idealized ISA, the temperature of the actual atmosphere does not always fall at a uniform rate with height. For example, there can be an inversion layer in which the temperature increases with altitude.Dry adiabatic lapse rate

Emagram diagram showing variation of dry adiabats (bold lines) and moist adiabats (dash lines) according to pressure and temperature
The dry adiabatic lapse rate (DALR) is the rate of temperature decrease with altitude for a parcel of dry or unsaturated air rising under adiabatic conditions. Unsaturated air has less than 100% relative humidity; i.e. its actual temperature is higher than its dew point. The term adiabatic means that no heat transfer occurs into or out of the parcel. Air has low thermal conductivity, and the bodies of air involved are very large, so transfer of heat by conduction is negligibly small.
Under these conditions when the air rises (for instance, by convection) it expands, because the pressure is lower at higher altitudes. As the air parcel expands, it pushes on the air around it, doing work (thermodynamics). Since the parcel does work but gains no heat, it loses internal energy so that its temperature decreases. The rate of temperature decrease is 9.8 °C/km (5.38 °F per 1,000 ft) (3.0 °C/1,000 ft). The reverse occurs for a sinking parcel of air.[7]
Since for adiabatic process:
 and :
and : we can show that:
we can show that: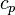 is the specific heat at constant pressure and
is the specific heat at constant pressure and  is the specific volume.
is the specific volume.Assuming an atmosphere in hydrostatic equilibrium:[8]
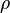 is the density. Combining these two equations to eliminate the pressure, one arrives at the result for the DALR,[9]
is the density. Combining these two equations to eliminate the pressure, one arrives at the result for the DALR,[9]Saturated adiabatic lapse rate
When the air is saturated with water vapour (at its dew point), the moist adiabatic lapse rate (MALR) or saturated adiabatic lapse rate (SALR) applies. This lapse rate varies strongly with temperature. A typical value is around 5 °C/km (2.7 °F/1,000 ft) (1.5 °C/1,000 ft).[citation needed]The reason for the difference between the dry and moist adiabatic lapse rate values is that latent heat is released when water condenses, thus decreasing the rate of temperature drop as altitude increases. This heat release process is an important source of energy in the development of thunderstorms. An unsaturated parcel of air of given temperature, altitude and moisture content below that of the corresponding dew point cools at the dry adiabatic lapse rate as altitude increases until the dew point line for the given moisture content is intersected. As the water vapour then starts condensing the air parcel subsequently cools at the slower moist adiabatic lapse rate if the altitude increases further.
The saturated adiabatic lapse rate is given approximately by this equation from the glossary of the American Meteorology Society:[10]
where: 
= Wet adiabatic lapse rate, K/m 
= Earth's gravitational acceleration = 9.8076 m/s2 
= Heat of vaporization of water, = 2501000 J/kg 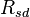
= Specific gas constant of dry air = 287 J kg−1 K−1 
= Specific gas constant of water vapour = 461.5 J kg−1 K−1 
=The dimensionless ratio of the specific gas constant of dry air to the specific gas constant for water vapour = 0.622 
= The water vapour pressure of the saturated air 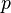
= The pressure of the saturated air 
= The mixing ratio of the mass of water vapour to the mass of dry air[11] 
= Temperature of the saturated air, K 
= The specific heat of dry air at constant pressure, = 1003.5 J kg−1 K−1
Thermodynamic-based lapse rate
Robert Essenhigh developed a comprehensive thermodynamic model of the lapse rate based on the Schuster–Schwarzschild (S–S) integral equations of transfer that govern radiation through the atmosphere including absorption and radiation by greenhouse gases.[12] His solution "predicts, in agreement with the Standard Atmosphere experimental data, a linear decline of the fourth power of the temperature, T4, with pressure, P, and, as a first approximation, a linear decline of T with altitude, h, up to the tropopause at about 10 km (the lower atmosphere)."[12] The predicted normalized density ratio and pressure ratio differ and fit the experimental data well.[citation needed] Sreekanth Kolan extended Essenhigh's model to include the energy balance for the lower and upper atmospheres.[13][self-published source?][third-party source needed]Significance in meteorology
The varying environmental lapse rates throughout the Earth's atmosphere are of critical importance in meteorology, particularly within the troposphere. They are used to determine if the parcel of rising air will rise high enough for its water to condense to form clouds, and, having formed clouds, whether the air will continue to rise and form bigger shower clouds, and whether these clouds will get even bigger and form cumulonimbus clouds (thunder clouds).As unsaturated air rises, its temperature drops at the dry adiabatic rate. The dew point also drops (as a result of decreasing air pressure) but much more slowly, typically about −2 °C per 1,000 m. If unsaturated air rises far enough, eventually its temperature will reach its dew point, and condensation will begin to form. This altitude is known as the lifting condensation level (LCL) when mechanical lift is present and the convective condensation level (CCL) when mechanical lift is absent, in which case, the parcel must be heated from below to its convective temperature. The cloud base will be somewhere within the layer bounded by these parameters.
The difference between the dry adiabatic lapse rate and the rate at which the dew point drops is around 8 °C per 1,000 ft. Given a difference in temperature and dew point readings on the ground, one can easily find the LCL by multiplying the difference by 125 m/°C.
If the environmental lapse rate is less than the moist adiabatic lapse rate, the air is absolutely stable — rising air will cool faster than the surrounding air and lose buoyancy. This often happens in the early morning, when the air near the ground has cooled overnight. Cloud formation in stable air is unlikely.
If the environmental lapse rate is between the moist and dry adiabatic lapse rates, the air is conditionally unstable — an unsaturated parcel of air does not have sufficient buoyancy to rise to the LCL or CCL, and it is stable to weak vertical displacements in either direction. If the parcel is saturated it is unstable and will rise to the LCL or CCL, and either be halted due to an inversion layer of convective inhibition, or if lifting continues, deep, moist convection (DMC) may ensue, as a parcel rises to the level of free convection (LFC), after which it enters the free convective layer (FCL) and usually rises to the equilibrium level (EL).
If the environmental lapse rate is larger than the dry adiabatic lapse rate, it has a superadiabatic lapse rate, the air is absolutely unstable — a parcel of air will gain buoyancy as it rises both below and above the lifting condensation level or convective condensation level. This often happens in the afternoon mainly over land masses. In these conditions, the likelihood of cumulus clouds, showers or even thunderstorms is increased.
Meteorologists use radiosondes to measure the environmental lapse rate and compare it to the predicted adiabatic lapse rate to forecast the likelihood that air will rise. Charts of the environmental lapse rate are known as thermodynamic diagrams, examples of which include Skew-T log-P diagrams and tephigrams.
The difference in moist adiabatic lapse rate and the dry rate is the cause of foehn wind phenomenon (also known as "Chinook winds" in parts of North America).

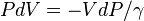



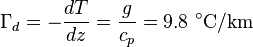










 , where
, where



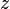 is the elevation,
is the elevation,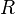 is the
is the  is the pressure at a given point at elevation
is the pressure at a given point at elevation  is pressure at the reference point = 101325 Pa at sea level.
is pressure at the reference point = 101325 Pa at sea level.
 is the atmospheric lapse rate (change in temperature / distance) = -6.5e-3 K/m, and
is the atmospheric lapse rate (change in temperature / distance) = -6.5e-3 K/m, and is the temperature at the same reference point for which
is the temperature at the same reference point for which 



 is the
is the  is the area of the surface on contact.
is the area of the surface on contact.


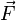 to the
to the  via
via
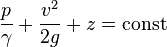
 = pressure head
= pressure head = velocity head
= velocity head

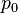 is the
is the  is the flow velocity
is the flow velocity
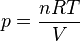
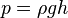


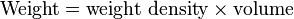




 , where h is the depth below the free surface.
, where h is the depth below the free surface.
 constant mass density. The SI unit of P is m2/s2. Kinematic pressure is used in the same manner as
constant mass density. The SI unit of P is m2/s2. Kinematic pressure is used in the same manner as  in order to compute
in order to compute