From Wikipedia, the free encyclopedia
Nanomaterials describe, in principle, materials of which a single unit is sized (in at least one dimension) between 1 to 1000
nanometres (10
−9 meter) but usually is 1 to 100 nm (the usual definition of
nanoscale[1]).
Nanomaterials research takes a
materials science-based approach to
nanotechnology, leveraging advances in materials
metrology and synthesis which have been developed in support of
microfabrication research. Materials with structure at the nanoscale often have unique optical, electronic, or mechanical properties.
[2]
Nanomaterials are slowly becoming commercialized
[3] and beginning to emerge as commodities.
[4]
Definition
There are significant differences among agencies on the definition of a nanomaterial.
[5]
In
ISO/TS 80004,
nanomaterial
is defined as a "material with any external dimension in the nanoscale
or having internal structure or surface structure in the nanoscale",
with
nanoscale defined as the "length range approximately from 1 nm to 100 nm". This includes both
nano-objects, which are discrete pieces of material, and
nanostructured materials, which have internal or surface structure on the nanoscale; a nanomaterial may be a member of both these categories.
[6]
On 18 October 2011, the
European Commission
adopted the following definition of a nanomaterial: "A natural,
incidental or manufactured material containing particles, in an unbound
state or as an aggregate or as an agglomerate and for 50% or more of the
particles in the number size distribution, one or more external
dimensions is in the size range 1 nm – 100 nm. In specific cases and
where warranted by concerns for the environment, health, safety or
competitiveness the number size distribution threshold of 50% may be
replaced by a threshold between 1% to 50%."
[7]
Sources
Engineered
Engineered nanomaterials have been deliberately engineered and manufactured by humans to have certain required properties.
[8]
Legacy nanomaterials are those that were in commercial production
prior to the development of nanotechnology as incremental advancements
over other
colloidal or particulate materials.
[9][10][11] They include
carbon black and
titanium dioxide nanoparticles.
[12]
Incidental
Nanomaterials
may be incidentally produced as a byproduct of mechanical or industrial
processes.Sources of incidental nanoparticles include vehicle engine
exhausts, welding fumes, combustion processes from domestic solid fuel
heating and cooking. Incidental atmospheric nanoparticles are often
referred to as
ultrafine particles, and are a contributor to
air pollution.
[13]
Natural
Biological systems often feature natural, functional nanomaterials. The structure of
foraminifera (mainly chalk) and viruses (protein,
capsid), the wax crystals covering a
lotus or
nasturtium leaf, spider and spider-mite silk,
[14] the blue hue of tarantulas,
[15] the "spatulae" on the bottom of
gecko feet, some
butterfly wing scales, natural colloids (
milk,
blood), horny materials (
skin,
claws,
beaks,
feathers,
horns,
hair),
paper,
cotton,
nacre,
corals, and even our own
bone matrix are all natural
organic nanomaterials.
Natural
inorganic nanomaterials occur through crystal growth in the diverse chemical conditions of the
Earth's crust. For example,
clays display complex nanostructures due to anisotropy of their underlying crystal structure, and volcanic activity can give rise to
opals, which are an instance of a naturally occurring
photonic crystals due to their nanoscale structure. Fires represent particularly complex reactions and can produce
pigments,
cement,
fumed silica etc.
Natural sources of nanoparticles include combustion products forest
fires, volcanic ash, ocean spray, and the radioactive decay of
radon gas. Natural nanomaterials can also be formed through weathering processes of metal- or anion-containing rocks, as well as at
acid mine drainage sites.
[13]
- Gallery of natural nanomaterials
-
-
"
Lotus effect", hydrophobic effect with self-cleaning ability
-
Close-up of the underside of a gecko's foot as it walks on a glass wall. (spatula: 200 × 10-15 nm).
-
SEM micrograph of a butterfly wing scale (× 5000)
-
-
Brazilian Crystal Opal. The play of color is caused by the
interference and diffraction of light between silica spheres (150 -
300 nm in diameter).
-
Blue hue of a species of
tarantula (450 nm ± 20 nm)
Types
Nano-objects are often categorized as to how many of their dimensions fall in the nanoscale. A
nanoparticle
is defined a nano-object with all three external dimensions in the
nanoscale, whose longest and the shortest axes do not differ
significantly. A
nanofiber has two external dimensions in the nanoscale, with
nanotubes being hollow nanofibers and
nanorods being solid nanofibers. A
nanoplate has one external dimension in the nanoscale, and if the two larger dimensions are significantly different it is called a
nanoribbon.
For nanofibers and nanoplates, the other dimensions may or may not be
in the nanoscale, but must be significantly larger. A significant
different in all cases is noted to be typically at least a factor of 3.
[16]
Nanostructured materials are often categorized by what
phases of matter the contain. A
nanocomposite
is a solid containing at least one physically or chemically distinct
region, or collection of regions, having at least one dimension in the
nanoscale.. A
nanofoam has a liquid or solid matrix, filled with a gaseous phase, where either phase has dimensions on the nanoscale. A
nanoporous material is a solid material containing
nanopores, cavities with dimensions on the nanoscale. A
nanocrystalline material has a significant fraction of crystal grains in the nanoscale.
[17]
The above definitions are all according to
ISO/TS 80004.
In other sources, nanoporous materials and nanofoam are sometimes
considered nanostructures but not nanomaterials because only the voids
and not the materials themselves are nanoscale.
[18] Although the ISO definition only considers round nano-objects to be
nanoparticles, other sources use the term nanoparticle for all shapes.
[19]
Nanoparticles
Nanoparticles have all three dimensions on the nanoscale.
Nanoparticles can also be embedded in a bulk solid to form a
nanocomposite.
[18]
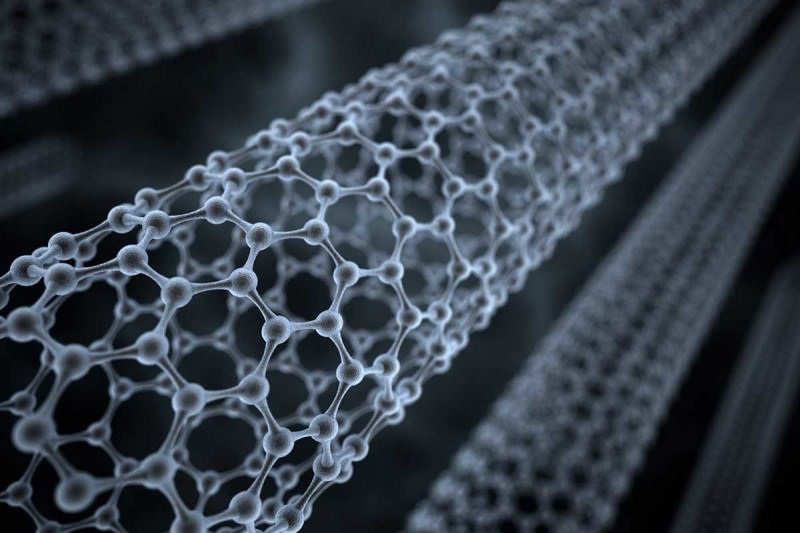
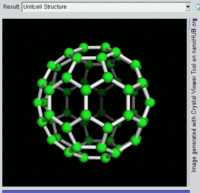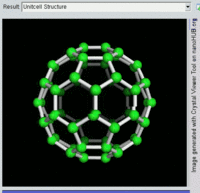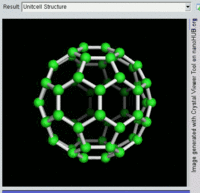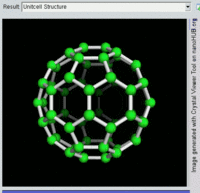
Fullerenes
The fullerenes are a class of
allotropes of carbon which conceptually are
graphene sheets rolled into tubes or spheres. These include the
carbon nanotubes (or
silicon nanotubes) which are of interest both because of their mechanical strength and also because of their electrical properties.
[20]
Rotating view of C60, one kind of fullerene.
The first fullerene molecule to be discovered, and the family's namesake,
buckminsterfullerene (C
60), was prepared in 1985 by
Richard Smalley,
Robert Curl,
James Heath,
Sean O'Brien, and
Harold Kroto at
Rice University. The name was a homage to
Buckminster Fuller, whose
geodesic domes it resembles. Fullerenes have since been found to occur in nature.
[21] More recently, fullerenes have been detected in outer space.
[22]
For the past decade, the chemical and physical properties of
fullerenes have been a hot topic in the field of research and
development, and are likely to continue to be for a long time. In April
2003, fullerenes were under study for
potential medicinal use: binding specific
antibiotics to the structure of resistant
bacteria and even target certain types of
cancer cells such as
melanoma. The October 2005 issue of Chemistry and Biology contains an article describing the use of fullerenes as light-activated
antimicrobial agents. In the field of
nanotechnology, heat resistance and
superconductivity are among the properties attracting intense research.
A common method used to produce fullerenes is to send a large current
between two nearby graphite electrodes in an inert atmosphere. The
resulting
carbon plasma arc between the electrodes cools into sooty residue from which many fullerenes can be isolated.
There are many calculations that have been done using ab-initio Quantum Methods applied to fullerenes. By
DFT and TDDFT methods one can obtain
IR,
Raman and
UV spectra. Results of such calculations can be compared with experimental results.
Metal-based nanoparticles
Inorganic nanomaterials, (e.g.
quantum dots,
nanowires and
nanorods) because of their interesting
optical and electrical properties, could be used in
optoelectronics.
[23]
Furthermore, the optical and electronic properties of nanomaterials
which depend on their size and shape can be tuned via synthetic
techniques. There are the possibilities to use those materials in
organic material based optoelectronic devices such as
Organic solar cells,
OLEDs etc. The operating principles of such devices are governed by photoinduced processes like
electron transfer
and energy transfer. The performance of the devices depends on the
efficiency of the photoinduced process responsible for their
functioning. Therefore, better understanding of those photoinduced
processes in organic/inorganic nanomaterial composite systems is
necessary in order to use them in optoelectronic devices.
Nanoparticles or
nanocrystals
made of metals, semiconductors, or oxides are of particular interest
for their mechanical, electrical, magnetic, optical, chemical and other
properties.
[24][25] Nanoparticles have been used as
quantum dots and as chemical
catalysts such as
nanomaterial-based catalysts. Recently, a range of nanoparticles are extensively investigated for
biomedical applications including
tissue engineering,
drug delivery,
biosensor.
[26] [27]
Nanoparticles are of great scientific interest as they are effectively a bridge between bulk materials and
atomic or
molecular
structures. A bulk material should have constant physical properties
regardless of its size, but at the nano-scale this is often not the
case. Size-dependent properties are observed such as
quantum confinement in
semiconductor particles,
surface plasmon resonance in some metal particles and
superparamagnetism in
magnetic materials.
Nanoparticles exhibit a number of special properties relative to bulk material. For example, the bending of bulk
copper (wire, ribbon, etc.) occurs with movement of copper atoms/clusters at about the 50 nm scale.
Copper nanoparticles smaller than 50 nm are considered super hard materials that do not exhibit the same
malleability and
ductility
as bulk copper. The change in properties is not always desirable.
Ferroelectric materials smaller than 10 nm can switch their
magnetisation direction using room temperature thermal energy, thus
making them useless for memory storage.
Suspensions of nanoparticles are possible because the interaction of the particle surface with the
solvent is strong enough to overcome differences in
density,
which usually result in a material either sinking or floating in a
liquid. Nanoparticles often have unexpected visual properties because
they are small enough to confine their electrons and produce quantum
effects. For example,
gold nanoparticles appear deep red to black in solution.
The often very high surface area to volume ratio of nanoparticles provides a tremendous driving force for
diffusion, especially at elevated temperatures.
Sintering
is possible at lower temperatures and over shorter durations than for
larger particles. This theoretically does not affect the density of the
final product, though flow difficulties and the tendency of
nanoparticles to agglomerate do complicate matters. The surface effects
of nanoparticles also reduces the incipient
melting temperature.
One-dimensional nanostructures
The
smallest possible crystalline wires with cross-section as small as a
single atom can be engineered in cylindrical confinement
[28][29][30].
Carbon nanotubes,
a natural semi-1D nanostructure, can be used as a template for
synthesis. Confinement provides mechanical stabilization and prevents
linear atomic chains from disintegration; other structures of 1D
nanowires are predicted to be mechanically stable even upon isolation from the templates
[31][32].
Two-dimensional nanostructures
2D materials are crystalline materials consisting of a two-dimensional single layer of atoms. The most important representative
graphene was discovered in 2004.
Box-shaped graphene (BSG)
nanostructure is an example of 3D nanomaterial.
[33] BSG nanostructure has appeared after mechanical cleavage of
pyrolytic graphite.
This nanostructure is a multilayer system of parallel hollow
nanochannels located along the surface and having quadrangular
cross-section. The thickness of the channel walls is approximately equal
to 1 nm. The typical width of channel facets makes about 25 nm.
Thin films
with nanoscale thicknesses are considered nanostructures, but are
sometimes not considered nanomaterials because they do not exist
separately from the substrate.
[18]
Bulk nanostructured materials
Some bulk materials contain features on the nanoscale, including
nanocomposites,
nanocrystalline materials,
nanostructured films, and
nanotextured surfaces.
[18]
Applications
Nano materials are used in a variety of, manufacturing processes,
products and healthcare including paints, filters, insulation and
lubricant additives. In healthcare
Nanozymes are nanomaterials with enzyme-like characteristics.
[34] They are an emerging type of
artificial enzyme, which have been used for wide applications in such as biosensing, bioimaging, tumor diagnosis,
[35] antibiofouling and more. In paints nanomaterials are used to improve UV protection and improve ease of cleaning.
[36] High quality filters may be produced using nanostructures, these
filters are capable of removing particulate as small as a virus as seen
in a water filter created by Seldon Technologies. In the air
purification field, nano technology was used to combat the spread of
MERS in Saudi Arabian hospitals in 2012.
[37]
Nanomaterials are being used in modern and human-safe insulation
technologies, in the past they were found in Asbestos-based insulation.
[38]
As a lubricant additive, nano materials have the ability to reduce
friction in moving parts. Worn and corroded parts can also be repaired
with self-assembling anisotropic nanoparticles called TriboTEX.
[37]
Synthesis
The
goal of any synthetic method for nanomaterials is to yield a material
that exhibits properties that are a result of their characteristic
length scale being in the nanometer range (1 – 100 nm).
Accordingly, the
synthetic method should exhibit control of size in this range so that
one property or another can be attained. Often the methods are divided
into two main types "Bottom Up" and "Top Down."
Bottom up methods
Bottom
up methods involve the assembly of atoms or molecules into
nanostructured arrays. In these methods the raw material sources can be
in the form of gases, liquids or solids. The latter requiring some sort
of disassembly prior to their incorporation onto a nanostructure. Bottom
methods generally fall into two categories: chaotic and controlled.
Chaotic processes involve elevating the constituent atoms or
molecules to a chaotic state and then suddenly changing the conditions
so as to make that state unstable. Through the clever manipulation of
any number of parameters, products form largely as a result of the
insuring kinetics. The collapse from the chaotic state can be difficult
or impossible to control and so ensemble statistics often govern the
resulting size distribution and average size. Accordingly, nanoparticle
formation is controlled through manipulation of the end state of the
products. Examples of Chaotic Processes are: Laser ablation, Exploding
wire, Arc, Flame pyrolysis, Combustion, Precipitation synthesis
techniques.
Controlled Processes involve the controlled delivery of the
constituent atoms or molecules to the site(s) of nanoparticle formation
such that the nanoparticle can grow to a prescribed sizes in a
controlled manner. Generally the state of the constituent atoms or
molecules are never far from that needed for nanoparticle formation.
Accordingly, nanoparticle formation is controlled through the control of
the state of the reactants. Examples of controlled processes are
self-limiting growth solution,
self-limited chemical vapor deposition, shaped pulse femtosecond laser techniques, and
molecular beam epitaxy.
Characterization
Novel effects can occur in materials when structures are formed with sizes comparable to any one of many possible
length scales, such as the
de Broglie wavelength of electrons, or the optical wavelengths of high energy photons. In these cases
quantum mechanical effects can dominate material properties. One example is
quantum confinement
where the electronic properties of solids are altered with great
reductions in particle size. The optical properties of nanoparticles,
e.g.
fluorescence,
also become a function of the particle diameter. This effect does not
come into play by going from macrosocopic to micrometer dimensions, but
becomes pronounced when the nanometer scale is reached.
In addition to optical and electronic properties, the novel mechanical properties of many nanomaterials is the subject of
nanomechanics
research. When added to a bulk material, nanoparticles can strongly
influence the mechanical properties of the material, such as the
stiffness or elasticity. For example, traditional
polymers can be reinforced by nanoparticles (such as
carbon nanotubes) resulting in novel materials which can be used as lightweight replacements for metals. Such
composite materials may enable a weight reduction accompanied by an increase in stability and improved functionality.
[39]
Finally, nanostructured materials with small particle size such as
zeolites, and
asbestos, are used as
catalysts
in a wide range of critical industrial chemical reactions. The further
development of such catalysts can form the basis of more efficient,
environmentally friendly chemical processes.
The first observations and size measurements of nano-particles were
made during the first decade of the 20th century. Zsigmondy made
detailed studies of gold sols and other nanomaterials with sizes down to
10 nm and less. He published a book in 1914.
[40] He used an
ultramicroscope that employs a
dark field method for seeing particles with sizes much less than
light wavelength.
There are traditional techniques developed during 20th century in
Interface and Colloid Science for characterizing nanomaterials. These are widely used for
first generation passive nanomaterials specified in the next section.
These methods include several different techniques for characterizing
particle size distribution.
This characterization is imperative because many materials that are
expected to be nano-sized are actually aggregated in solutions. Some of
methods are based on
light scattering. Others apply
ultrasound, such as
ultrasound attenuation spectroscopy for testing concentrated nano-dispersions and microemulsions.
[41]
There is also a group of traditional techniques for characterizing
surface charge or
zeta potential of nano-particles in solutions. This information is required for proper system stabilzation, preventing its aggregation or
flocculation. These methods include
microelectrophoresis,
electrophoretic light scattering and
electroacoustics. The last one, for instance
colloid vibration current method is suitable for characterizing concentrated systems.
Uniformity
The
chemical processing and synthesis of high performance technological
components for the private, industrial and military sectors requires the
use of high purity
ceramics,
polymers,
glass-ceramics and material
composites. In condensed bodies formed from fine powders, the irregular sizes and shapes of
nanoparticles
in a typical powder often lead to non-uniform packing morphologies that
result in packing density variations in the powder compact.
Uncontrolled
agglomeration of powders due to
attractive van der Waals forces
can also give rise to in microstructural inhomogeneities. Differential
stresses that develop as a result of non-uniform drying shrinkage are
directly related to the rate at which the
solvent can be removed, and thus highly dependent upon the distribution of
porosity. Such stresses have been associated with a plastic-to-brittle transition in consolidated bodies, and can yield to
crack propagation in the unfired body if not relieved.
[42][43] [44]
In addition, any fluctuations in packing density in the compact as it is prepared for the kiln are often amplified during the
sintering process, yielding inhomogeneous densification. Some pores and other structural
defects
associated with density variations have been shown to play a
detrimental role in the sintering process by growing and thus limiting
end-point densities. Differential stresses arising from inhomogeneous
densification have also been shown to result in the propagation of
internal cracks, thus becoming the strength-controlling flaws.
[45][46]
It would therefore appear desirable to process a material in such a
way that it is physically uniform with regard to the distribution of
components and porosity, rather than using particle size distributions
which will maximize the green density. The containment of a uniformly
dispersed assembly of strongly interacting particles in suspension
requires total control over particle-particle interactions. It should be
noted here that a number of dispersants such as ammonium citrate
(aqueous) and imidazoline or
oleyl alcohol (nonaqueous) are promising solutions as possible additives for enhanced dispersion and deagglomeration.
Monodisperse nanoparticles and colloids provide this potential.
[47]
Monodisperse powders of colloidal
silica, for example, may therefore be stabilized sufficiently to ensure a high degree of order in the
colloidal crystal or
polycrystalline
colloidal solid which results from aggregation. The degree of order
appears to be limited by the time and space allowed for longer-range
correlations to be established. Such defective polycrystalline colloidal
structures would appear to be the basic elements of sub-micrometer
colloidal materials science, and, therefore, provide the first step in
developing a more rigorous understanding of the mechanisms involved in
microstructural evolution in high performance materials and components.
Health and safety
World Health Organization guidelines
The
World Health Organization (WHO) published a guideline on protecting
workers from potential risk of manufactured nanomaterials at the end of
2017.
[50]
WHO used a precautionary approach as one of its guiding principles.
This means that exposure has to be reduced, despite uncertainty about
the adverse health effects, when there are reasonable indications to do
so. In addition, the hierarchy of controls was an important guiding
principle. This means that when there is a choice between control
measures, those measures that are closer to the root of the problem
should always be preferred over measures that put a greater burden on
workers, such as the use of personal protective equipment (PPE). WHO
commissioned systematic reviews for all important issues to assess the
current state of the science and to inform the recommendations according
to the process set out in the WHO Handbook for guideline development.
The recommendations were rated as “strong” or “conditional” depending on
the quality of the scientific evidence, values and preferences, and
costs related to the recommendation.
The WHO guidelines contain the following recommendations for safe handling of MNMs:
A. Assess health hazards of MNMs
- WHO recommends assigning hazard classes to all MNMs according to the
Globally Harmonized System (GHS) of Classification and Labelling of
Chemicals for use in safety data sheets. For a limited number of MNMs
this information is made available in the guidelines (strong
recommendation, moderate-quality evidence).
- WHO recommends updating safety data sheets with MNM-specific hazard
information or indicating which toxicological end-points did not have
adequate testing available (strong recommendation, moderate-quality
evidence).
- For the respirable fibres and granular biopersistent particles’
groups, the GDG suggests using the available classification of MNMs for
provisional classification of nanomaterials of the same group
(conditional recommendation, low-quality evidence).
B. Assess exposure to MNMs
- WHO suggests assessing workers’ exposure in workplaces with methods
similar to those used for the proposed specific occupational exposure
limit (OEL) value of the MNM (conditional recommendation, low-quality
evidence).
- Because there are no specific regulatory OEL values for MNMs in
workplaces, WHO suggests assessing whether workplace exposure exceeds a
proposed OEL value for the MNM. A list of proposed OEL values is
provided in an annex of the guidelines. The chosen OEL should be at
least as protective as a legally mandated OEL for the bulk form of the
material (conditional recommendation, low-quality evidence).
- If specific OELs for MNMs are not available in workplaces, WHO
suggests a step-wise approach for inhalation exposure with, first an
assessment of the potential for exposure; second, conducting basic
exposure assessment and third, conducting a comprehensive exposure
assessment such as those proposed by the Organisation for Economic
Cooperation and Development (OECD) or Comité Européen de Normalisation
(the European Committee for Standardization, CEN) (conditional
recommendation, moderate quality evidence).
- For dermal exposure assessment, WHO found that there was
insufficient evidence to recommend one method of dermal exposure
assessment over another.
C. Control exposure to MNMs
- Based on a precautionary approach, WHO recommends focusing control
of exposure on preventing inhalation exposure with the aim of reducing
it as much as possible (strong recommendation, moderate-quality
evidence).
- WHO recommends reduction of exposures to a range of MNMs that have
been consistently measured in workplaces especially during cleaning and
maintenance, collecting material from reaction vessels and feeding MNMs
into the production process. In the absence of toxicological
information, WHO recommends implementing the highest level of controls
to prevent workers from any exposure. When more information is
available, WHO recommends taking a more tailored approach (strong
recommendation, moderate-quality evidence).
- WHO recommends taking control measures based on the principle of
hierarchy of controls, meaning that the first control measure should be
to eliminate the source of exposure before implementing control measures
that are more dependent on worker involvement, with PPE being used only
as a last resort. According to this principle, engineering controls
should be used when there is a high level of inhalation exposure or when
there is no, or very little, toxicological information available. In
the absence of appropriate engineering controls PPE should be used,
especially respiratory protection, as part of a respiratory protection
programme that includes fit-testing (strong recommendation,
moderate-quality evidence).
- WHO suggests preventing dermal exposure by occupational hygiene
measures such as surface cleaning, and the use of appropriate gloves
(conditional recommendation, low quality evidence).
- When assessment and measurement by a workplace safety expert is not
available, WHO suggests using control banding for nanomaterials to
select exposure control measures in the workplace. Owing to a lack of
studies, WHO cannot recommend one method of control banding over another
(conditional recommendation, very low-quality evidence).
For health surveillance WHO could not make a recommendation for
targeted MNM-specific health surveillance programmes over existing
health surveillance programmes that are already in use owing to the lack
of evidence. WHO considers training of workers and worker involvement
in health and safety issues to be best practice but could not recommend
one form of training of workers over another, or one form of worker
involvement over another, owing to the lack of studies available. It is
expected that there will be considerable progress in validated
measurement methods and risk assessment and WHO expects to update these
guidelines in five years’ time, in 2022.
Other guidance
Because
nanotechnology is a recent development, the health and safety effects
of exposures to nanomaterials, and what levels of exposure may be
acceptable, are subjects of ongoing research.
[8] Of the possible hazards,
inhalation exposure appears to present the most concern. Animal studies indicate that
carbon nanotubes and
carbon nanofibers can cause pulmonary effects including
inflammation,
granulomas, and
pulmonary fibrosis, which were of similar or greater potency when compared with other known
fibrogenic materials such as
silica,
asbestos, and ultrafine
carbon black. Although the extent to which animal data may predict clinically
significant lung effects in workers is not known, the toxicity seen in
the short-term animal studies indicate a need for protective action for
workers exposed to these nanomaterials, although no reports of actual
adverse health effects in workers using or producing these nanomaterials
were known as of 2013.
[51] Additional concerns include skin contact and ingestion exposure,
[51][52][53] and
dust explosion hazards.
[54][55]
Elimination and
substitution are the most desirable approaches to
hazard control. While the nanomaterials themselves often cannot be eliminated or substituted with conventional materials,
[8] it may be possible to choose properties of the nanoparticle such as
size,
shape,
functionalization,
surface charge,
solubility,
agglomeration, and
aggregation state to improve their toxicological properties while retaining the desired functionality.
[56] Handling procedures can also be improved, for example, using a nanomaterial
slurry or
suspension in a liquid solvent instead of a dry powder will reduce dust exposure.
[8] Engineering controls are physical changes to the workplace that isolate workers from hazards, mainly ventilation systems such as
fume hoods,
gloveboxes,
biosafety cabinets, and
vented balance enclosures.
[57] Administrative controls are changes to workers' behavior to mitigate a hazard, including training on
best practices
for safe handling, storage, and disposal of nanomaterials, proper
awareness of hazards through labeling and warning signage, and
encouraging a general
safety culture.
Personal protective equipment must be worn on the worker's body and is the least desirable option for controlling hazards.
[8]
Personal protective equipment normally used for typical chemicals are
also appropriate for nanomaterials, including long pants, long-sleeve
shirts, and closed-toed shoes, and the use of
safety gloves,
goggles, and impervious
laboratory coats.
[57] In some circumstances
respirators may be used.
[56]
Exposure assessment
is a set of methods used to monitor contaminant release and exposures
to workers. These methods include personal sampling, where samplers are
located in the personal breathing zone of the worker, often attached to a
shirt collar to be as close to the nose and mouth as possible; and
area/background sampling, where they are placed at static locations. The
assessment should use both
particle counters,
which monitor the real-time quantity of nanomaterials and other
background particles; and filter-based samples, which can be used to
identify the nanomaterial, usually using
electron microscopy and
elemental analysis.
[56][58] As of 2016, quantitative
occupational exposure limits have not been determined for most nanomaterials. The U.S.
National Institute for Occupational Safety and Health has determined non-regulatory
recommended exposure limits for
carbon nanotubes,
carbon nanofibers,
[51] and
ultrafine titanium dioxide.
[59] Agencies and organizations from other countries, including the
British Standards Institute[60] and the
Institute for Occupational Safety and Health in Germany,
[61] have established OELs for some nanomaterials, and some companies have supplied OELs for their products.
[8]