From Wikipedia, the free encyclopedia
| Liver | |
|---|---|

Human liver shown in abdomen
|
|
| Details | |
| Latin | Jecur, iecur |
| Greek | Hepar (ἧπαρm) root hepat- (ἡπατ-) |
| Precursor | Foregut |
| System | Digestive system |
| hepatic artery | |
| Hepatic vein and hepatic portal vein | |
| Celiac ganglia and vagus nerve[1] | |
| Identifiers | |
| Gray's | p.1188 |
| MeSH | A03.620 |
| TA | A05.8.01.001 |
| FMA | 7197 |
| Anatomical terminology | |
The liver is a vital organ of vertebrates and some other animals. In the human it is located in the upper right quadrant of the abdomen, below the diaphragm. The liver has a wide range of functions, including detoxification of various metabolites, protein synthesis, and the production of biochemicals necessary for digestion. There is currently no way to compensate for the absence of liver function in the long term, although liver dialysis techniques can be used in the short term.
The liver is a gland and plays a major role in metabolism with numerous functions in the human body, including regulation of glycogen storage, decomposition of red blood cells, plasma protein synthesis, hormone production, and detoxification. It is an accessory digestive gland and produces bile, an alkaline compound which aids in digestion via the emulsification of lipids. The liver's highly specialized tissue consisting of mostly hepatocytes regulates a wide variety of high-volume biochemical reactions, including the synthesis and breakdown of small and complex molecules, many of which are necessary for normal vital functions.[2] Estimates regarding the organ's total number of functions vary, but textbooks generally cite it being around 500.[3]
Terminology related to the liver often starts in hepar- or hepat- from the Greek word for liver, hēpar (ἧπαρ, root hepat-, ἡπατ-).[4][5]
Structure
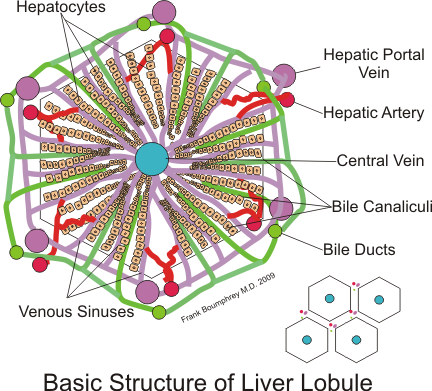
The liver is a reddish brown triangular organ with four lobes of unequal size and shape. A human liver normally weighs 1.44–1.66 kg (3.2–3.7 lb).[6] It is both the largest internal organ and the largest gland in the human body. Located in the right upper quadrant of the abdominal cavity, it rests just below the diaphragm, to the right of the stomach and overlying the gallbladder. It is connected to two large blood vessels, the hepatic artery and the portal vein. The hepatic artery carries oxygen-rich blood from the aorta, whereas the portal vein carries blood rich in digested nutrients from the entire gastrointestinal tract and also from the spleen and pancreas. These blood vessels subdivide into small capillaries known as liver sinusoids, which then lead to a lobule. Lobules are the functional units of the liver and each lobule is made up of millions of hepatic cells (hepatocytes) which are the basic metabolic cells.
Gross anatomy
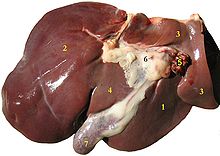
Gross anatomy traditionally divided the liver into two – a right and a left lobe, as viewed from the front (diaphragmatic) surface; but the underside (the visceral surface) shows it to be divided into four lobes and includes the caudate and quadrate lobes.
The falciform ligament is visible on the front of the liver and this divides the liver into a left and a much larger right lobe. From the visceral surface, the two additional lobes are located between the right and left lobes, one in front of the other. On the visceral surface a functional anatomy determines the organisation of the liver. A line can be visualised running from the left of the vena cava and all the way forward to divide the liver and gallbladder into two halves. This line is called Cantlie's line. Other anatomical landmarks exist, such as the ligamentum venosum and the round ligament of the liver (ligamentum teres) that further divide the left side of the liver in two sections. Now an important anatomical landmark, the transverse fissure of the liver divides this left portion of the liver into four segments which will be numbered starting at the caudate lobule as I in an anti-clock manner. From this visceral view we can see 7 segments because the 8th segment is only visible in the parietal view.
Surfaces
On the diaphragmatic surface, apart from a large triangular bare area, where it connects to the diaphragm, the liver is covered by a thin double-layered membrane, the peritoneum, that reduces friction against other organs. This surface covers the convex shape of the two lobes where it accommodates the shape of the diaphragm. The peritoneum folds back on itself to form the falciform ligament and the right and left triangular ligaments.These "peritoneal ligaments" are not related to the true anatomic ligaments in joints, and the right and left triangular ligaments have no known functional importance, but they serve as recognizable surface landmarks. The falciform ligament however, does function to attach the liver to the posterior portion of the anterior body wall.
The visceral surface or inferior surface, is uneven and concave. It is covered in peritoneum apart from where it attaches the gallbladder and the porta hepatis.
Impressions
There are several impressions on the surface of the liver which accommodate the various adjacent structures and organs. Underneath the right lobe and to the right of the gallbladder fossa, are two impressions, one behind the other and separated by a ridge. The one in front is a shallow colic impression, formed by the hepatic flexure and the one behind is a deeper renal impression accommodating part of the right kidney and part of the suprarenal gland.
The suprarenal impression is a small triangular depressed area on the liver. It is located close to the right of the fossa between the bare area and the caudate lobe and immediately above the renal impression. The greater part of the suprarenal impression is devoid of peritoneum and it lodges the right suprarenal gland.
Medial to the renal impression is a third and slightly marked impression, lying between it and the neck of the gall-bladder. This is caused by the descending portion of the duodenum, and is known as the duodenal impression.
The inferior surface of the left lobe of the liver presents behind and to the left the gastric impression, moulded over the antero-superior surface of the stomach, and to the right of this a rounded eminence, the tuber omentale, which fits into the concavity of the lesser curvature of the stomach and lies in front of the anterior layer of the lesser omentum.
Microscopic anatomy
Histology, the study of microscopic anatomy shows two major types of cells of the liver: parenchymal and non-parenchymal cells. 80% of the liver volume is occupied by parenchymal cells commonly referred to as hepatocytes. Non-parenchymal cells constitute 40% of the total number of liver cells but only 6.5% of its volume. The liver sinusoids are lined with two types of cell, sinusoidal endothelial cells, and phagocytic Kupffer cells.[7] Hepatic stellate cells are some of the non-parenchymal cells are external to the sinusoid in the space of Disse.[8]
Each of the lobes is seen to be made up of hepatic lobules; a vein goes from the centre, which then joins to the hepatic vein to carry blood out from the liver. On the surface of the lobules, there are ducts, veins and arteries that carry fluids to and from them. A distinctive component of a lobule is the portal triad.
Functional anatomy
| Segment* | Couinaud segments |
|---|---|
| Caudate | 1 |
| Lateral | 2, 3 |
| Medial | 4a, 4b |
| Right | 5, 6, 7, 8 |
* or lobe, in the case of the caudate lobe
Each number in the list corresponds to one in the table 1. Caudate 2. Superior subsegment of the lateral segment 3. Inferior subsegment of the lateral segment 4a. Superior subsegment of the medial segment 4b. Inferior subsegment of the medial segment 5. Inferior subsegment of the anterior segment 6. Inferior subsegment of the posterior segment 7. Superior subsegment of the posterior segment 8. Superior subsegment of the anterior segment |
|
The functional lobes are separated by the imaginary plane, Cantlie's line joining the gallbladder fossa to the inferior vena cava. The plane separates the liver into the true right and left lobes. The middle hepatic vein also demarcates the true right and left lobes. The right lobe is further divided into an anterior and posterior segment by the right hepatic vein. The left lobe is divided into the medial and lateral segments by the left hepatic vein. The fissure for the round ligament of the liver (ligamentum teres) also separates the medial and lateral segments. The medial segment is also called the quadrate lobe. In the widely used Couinaud (or "French") system, the functional lobes are further divided into a total of eight subsegments based on a transverse plane through the bifurcation of the main portal vein. The caudate lobe is a separate structure which receives blood flow from both the right- and left-sided vascular branches.[9][10]
Development
Organogenesis, the development of the organs takes place from the third to the eighth week in human embryogenesis. The origins of the liver lie in both the ventral portion of the foregut endoderm (endoderm being one of the 3 embryonic germ cell layers) and the constituents of the adjacent septum transversum mesenchyme. In human embryo, the hepatic diverticulum is the tube of endoderm that extends out from the foregut into the surrounding mesenchyme. The mesenchyme of septum transversum induces this endoderm to proliferate, to branch, and to form the glandular epithelium of the liver. A portion of the hepatic diverticulum (that region closest to the digestive tube) continues to function as the drainage duct of the liver, and a branch from this duct produces the gallbladder.[11]Besides signals from the septum transversum mesenchyme, fibroblast growth factor from the developing heart also contributes to hepatic competence, along with retinoic acid emanating from the lateral plate mesoderm. The hepatic endodermal cells undergo a morphological transition from columnar to pseudostratified resulting in thickening into the early liver bud. Their expansion forms a population of the bipotential hepatoblasts.[12] Hepatic stellate cells are derived from mesenchyme.[13]
After migration of hepatoblasts into the septum transversum mesenchyme, the hepatic architecture begins to be established, with liver sinusoids and bile canaliculi appearing. The liver bud separates into the lobes. The left umbilical vein becomes the ductus venosus and the right vitelline vein becomes the portal vein. The expanding liver bud is colonized by hematopoietic cells. The bipotential hepatoblasts begin differentiating into biliary epithelial cells and hepatocytes. The biliary epithelial cells differentiate from hepatoblasts around portal veins, first producing a monolayer, and then a bilayer of cuboidal cells. In ductal plate, focal dilations emerge at points in the bilayer, become surrounded by portal mesenchyme, and undergo tubulogenesis into intrahepatic bile ducts. Hepatoblasts not adjacent to portal veins instead differentiate into hepatocytes and arrange into cords lined by sinudoidal epithelial cells and bile canaliculi. Once hepatoblasts are specified into hepatocytes and undergo further expansion, they begin acquiring the functions of a mature hepatocyte, and eventually mature hepatocytes appear as highly polarized epithelial cells with abundant glycogen accumulation. In the adult liver, hepatocytes are not equivalent, with position along the portocentrovenular axis within a liver lobule dictating expression of metabolic genes involved in drug metabolism, carbohydrate metabolism, ammonia detoxification, and bile production and secretion. WNT/β-catenin has now been identified to be playing a key role in this phenomenon.[12]
Fetal blood supply
In the growing fetus, a major source of blood to the liver is the umbilical vein which supplies nutrients to the growing fetus. The umbilical vein enters the abdomen at the umbilicus, and passes upward along the free margin of the falciform ligament of the liver to the inferior surface of the liver. There it joins with the left branch of the portal vein. The ductus venosus carries blood from the left portal vein to the left hepatic vein and then to the inferior vena cava, allowing placental blood to bypass the liver.In the fetus, the liver develops throughout normal gestation, and does not perform the normal filtration of the infant liver. The liver does not perform digestive processes because the fetus does not consume meals directly, but receives nourishment from the mother via the placenta. The fetal liver releases some blood stem cells that migrate to the fetal thymus, so initially the lymphocytes, called T-cells, are created from fetal liver stem cells. Once the fetus is delivered, the formation of blood stem cells in infants shifts to the red bone marrow.
After birth, the umbilical vein and ductus venosus are completely obliterated in two to five days; the former becomes the ligamentum teres and the latter becomes the ligamentum venosum. In the disease state of cirrhosis and portal hypertension, the umbilical vein can open up again.
During childhood
At birth the liver comprises roughly 4% of body weight and is at average 120g. Over the course of development it will increase to 1,4–1,6 kg but will only take up 2.5–3.5% of body weight.[14]Physiology
The various functions of the liver are carried out by the liver cells or hepatocytes. Currently, there is no artificial organ or device capable of emulating all the functions of the liver. Some functions can be emulated by liver dialysis, an experimental treatment for liver failure. The liver is thought to be responsible for up to 500 separate functions, usually in combination with other systems and organs.Blood supply
The liver gets a dual blood supply from the hepatic portal vein and hepatic arteries. Supplying approximately 75% of the liver's blood supply, the hepatic portal vein carries venous blood drained from the spleen, gastrointestinal tract, and its associated organs. The hepatic arteries supply arterial blood to the liver, accounting for the remainder of its blood flow. Oxygen is provided from both sources; approximately half of the liver's oxygen demand is met by the hepatic portal vein, and half is met by the hepatic arteries.[15]Blood flows through the liver sinusoids and empties into the central vein of each lobule. The central veins coalesce into hepatic veins, which leave the liver.
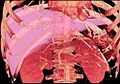
-
Axial CT image showing anomalous hepatic veins coursing on the subcapsular anterior surface of the liver.[16]
Biliary flow
The term biliary tree is derived from the branches of the bile ducts. The bile produced in the liver is collected in bile canaliculi, which merge to form bile ducts. Within the liver, these ducts are called intrahepatic (within the liver) bile ducts, and once they exit the liver they are considered extrahepatic (outside the liver). The intrahepatic ducts eventually drain into the right and left hepatic ducts, which merge to form the common hepatic duct. The cystic duct from the gallbladder joins with the common hepatic duct to form the common bile duct.
Bile either drains directly into the duodenum via the common bile duct, or is temporarily stored in the gallbladder via the cystic duct. The common bile duct and the pancreatic duct enter the second part of the duodenum together at the ampulla of Vater.
Synthesis
- A large part of amino acid synthesis
- The liver performs several roles in carbohydrate metabolism:
- Gluconeogenesis (the synthesis of glucose from certain amino acids, lactate or glycerol)
- Glycogenolysis (the breakdown of glycogen into glucose)
- Glycogenesis (the formation of glycogen from glucose)(muscle tissues can also do this)
- The liver is responsible for the mainstay of protein metabolism, synthesis as well as degradation.
- The liver also performs several roles in lipid metabolism:
- Cholesterol synthesis
- Lipogenesis, the production of triglycerides (fats).
- A bulk of the lipoproteins are synthesized in the liver.
- The liver produces coagulation factors I (fibrinogen), II (prothrombin), V, VII, VIII, IX, X and XI, as well as protein C, protein S and antithrombin.
- In the first trimester fetus, the liver is the main site of red blood cell production. By the 32nd week of gestation, the bone marrow has almost completely taken over that task.
- The liver produces and excretes bile (a yellowish liquid) required for emulsifying fats and help the absorption of vitamin K from the diet. Some of the bile drains directly into the duodenum, and some is stored in the gallbladder.
- The liver also produces insulin-like growth factor 1 (IGF-1), a polypeptide protein hormone that plays an important role in childhood growth and continues to have anabolic effects in adults.
- The liver is a major site of thrombopoietin production. Thrombopoietin is a glycoprotein hormone that regulates the production of platelets by the bone marrow.
Breakdown
- The breakdown of insulin and other hormones
- The liver glucoronidates bilirubin, facilitating its excretion into bile.
- The liver breaks down or modifies toxic substances (e.g., methylation) and most medicinal products in a process called drug metabolism. This sometimes results in toxication, when the metabolite is more toxic than its precursor. Preferably, the toxins are conjugated to avail excretion in bile or urine.
- The liver converts ammonia to urea (urea cycle).
Other functions
- The liver stores a multitude of substances, including glucose (in the form of glycogen), vitamin A (1–2 years' supply), vitamin D (1–4 months' supply)[citation needed], vitamin B12 (1–3 years' supply), vitamin K, iron, and copper.
- The liver is responsible for immunological effects—the reticuloendothelial system of the liver contains many immunologically active cells, acting as a 'sieve' for antigens carried to it via the portal system.
- The liver produces albumin, the major osmolar component of blood serum.
- The liver synthesizes angiotensinogen, a hormone that is responsible for raising the blood pressure when activated by renin, an enzyme that is released when the kidney senses low blood pressure.
Relation to medicine and pharmacology
The oxidative capacity of the liver decreases with aging and therefore any medications that require oxidation (for instance, benzodiazepines) are more likely to accumulate to toxic levels. However, medications with shorter half-lives, such as lorazepam and oxazepam, are preferred in most cases when benzodiazepines are required in regards to geriatric medicine.Clinical significance
Disease

The liver supports almost every organ in the body and is vital for survival. Because of its strategic location and multidimensional functions, the liver is also prone to many diseases.[17]
Hepatitis is a common condition of inflammation of the liver. The most usual cause of this is viral, and the most common of these infections are hepatitis A B C D and E. Some of these infections are sexually transmitted. Inflammation can also be caused by other viruses in the Herpesviridae family such as the herpes simplex virus. Infection with hepatitis B virus or hepatitis C virus is the main cause of liver cancer.
Other disorders caused by excessive alcohol consumption are grouped under alcoholic liver diseases and these include alcoholic hepatitis, fatty liver, and cirrhosis. Liver damage can also be caused by drugs in particular paracetomol and drugs used to treat cancer.
Many diseases of the liver are accompanied by jaundice caused by increased levels of bilirubin in the system. The bilirubin results from the breakup of the hemoglobin of dead red blood cells; normally, the liver removes bilirubin from the blood and excretes it through bile.
There are also many pediatric liver diseases including biliary atresia, alpha-1 antitrypsin deficiency, alagille syndrome, progressive familial intrahepatic cholestasis, and Langerhans cell histiocytosis, to name but a few.
Diseases that interfere with liver function will lead to derangement of these processes. However, the liver has a great capacity to regenerate and has a large reserve capacity. In most cases, the liver only produces symptoms after extensive damage.
Liver diseases may be diagnosed by liver function tests, for example, by production of acute phase proteins.
Symptoms
The classic symptoms of liver damage include the following:- Pale stools occur when stercobilin, a brown pigment, is absent from the stool. Stercobilin is derived from bilirubin metabolites produced in the liver.
- Dark urine occurs when bilirubin mixes with urine
- Jaundice (yellow skin and/or whites of the eyes) This is where bilirubin deposits in skin, causing an intense itch. Itching is the most common complaint by people who have liver failure. Often this itch cannot be relieved by drugs.
- Swelling of the abdomen, ankles and feet occurs because the liver fails to make albumin.
- Excessive fatigue occurs from a generalized loss of nutrients, minerals and vitamins.
- Bruising and easy bleeding are other features of liver disease. The liver makes substances which help prevent bleeding. When liver damage occurs, these substances are no longer present and severe bleeding can occur.[18]
Diagnosis
The diagnosis of liver function is made by liver function tests, groups of blood tests, that can readily show the extent of liver damage. If infection is suspected, then other serological tests will be carried out. Sometimes, an ultrasound or a CT scan is needed to produce an image of the liver.Physical examination of the liver can only reveal its size and any tenderness, and some form of imaging will also be needed.[19]
Biopsy / scan
Damage to the liver is sometimes determined with a biopsy, particularly when the cause of liver damage is unknown. In the 21st century they were largely replaced by high-resolution radiographic scans. The latter do not require ultrasound guidance, lab involvement, microscopic analysis, organ damage, pain, or patient sedation; and the results are available immediately on a computer screen.In a biopsy, a needle is inserted into the skin just below the rib cage and a tissue sample obtained. The tissue is sent to the laboratory, where it is analyzed under a microscope. Sometimes, a radiologist may assist the physician performing a liver biopsy by providing ultrasound guidance.[20]
Liver regeneration
The liver is the only human internal organ capable of natural regeneration of lost tissue; as little as 25% of a liver can regenerate into a whole liver.[21] This is, however, not true regeneration but rather compensatory growth in mammals.[22] The lobes that are removed do not regrow and the growth of the liver is a restoration of function, not original form. This contrasts with true regeneration where both original function and form are restored. In lower species such as fish, the liver undergoes true regeneration by restoring both shape and size of the organ.[23] In liver, large areas of the tissues are formed but for the formation of new cells there must be sufficient amount of material so the circulation of the blood becomes more active.[24]This is predominantly due to the hepatocytes re-entering the cell cycle. That is, the hepatocytes go from the quiescent G0 phase to the G1 phase and undergo mitosis. This process is activated by the p75 receptors.[25] There is also some evidence of bipotential stem cells, called hepatic oval cells or ovalocytes (not to be confused with oval red blood cells of ovalocytosis), which are thought to reside in the canals of Hering. These cells can differentiate into either hepatocytes or cholangiocytes, the latter being the cells that line the bile ducts.[citation needed]
Scientific and medical works about liver regeneration often refer to the Greek Titan Prometheus who was chained to a rock in the Caucasus where, each day, his liver was devoured by an eagle, only to grow back each night. The myth suggests the ancient Greeks knew about the liver’s remarkable capacity for self-repair, however, this claim is purely speculative.[26]
Liver transplantation
Human liver transplants were first performed by Thomas Starzl in the United States and Roy Calne in Cambridge, England in 1963 and 1965, respectively.Liver transplantation is the only option for those with irreversible liver failure. Most transplants are done for chronic liver diseases leading to cirrhosis, such as chronic hepatitis C, alcoholism, autoimmune hepatitis, and many others. Less commonly, liver transplantation is done for fulminant hepatic failure, in which liver failure occurs over days to weeks.
Liver allografts for transplant usually come from donors who have died from fatal brain injury. Living donor liver transplantation is a technique in which a portion of a living person's liver is removed and used to replace the entire liver of the recipient. This was first performed in 1989 for pediatric liver transplantation. Only 20 percent of an adult's liver (Couinaud segments 2 and 3) is needed to serve as a liver allograft for an infant or small child.
More recently, adult-to-adult liver transplantation has been done using the donor's right hepatic lobe, which amounts to 60 percent of the liver. Due to the ability of the liver to regenerate, both the donor and recipient end up with normal liver function if all goes well. This procedure is more controversial, as it entails performing a much larger operation on the donor, and indeed there have been at least two donor deaths out of the first several hundred cases. A recent publication has addressed the problem of donor mortality, and at least 14 cases have been found.[27] The risk of postoperative complications (and death) is far greater in right-sided operations than that in left-sided operations.
With the recent advances of noninvasive imaging, living liver donors usually have to undergo imaging examinations for liver anatomy to decide if the anatomy is feasible for donation. The evaluation is usually performed by multidetector row computed tomography (MDCT) and magnetic resonance imaging (MRI). MDCT is good in vascular anatomy and volumetry. MRI is used for biliary tree anatomy. Donors with very unusual vascular anatomy, which makes them unsuitable for donation, could be screened out to avoid unnecessary operations.
Society and culture
In Greek mythology, Prometheus was punished by the gods for revealing fire to humans, by being chained to a rock where a vulture (or an eagle) would peck out his liver, which would regenerate overnight. (The liver is the only human internal organ that actually can regenerate itself to a significant extent.) Many ancient peoples of the Near East and Mediterranean areas practiced a type of divination called haruspicy, where they tried to obtain information by examining the livers of sheep and other animals.In Plato, and in later physiology, the liver was thought to be the seat of the darkest emotions (specifically wrath, jealousy and greed) which drive men to action.[28] The Talmud (tractate Berakhot 61b) refers to the liver as the seat of anger, with the gallbladder counteracting this.
The Persian, Urdu, and Hindi languages (جگر or जिगर or jigar) refer to the liver in figurative speech to indicate courage and strong feelings, or "their best"; e.g., "This Mecca has thrown to you the pieces of its liver!".[29] The term jan e jigar, literally "the strength (power) of my liver", is a term of endearment in Urdu. In Persian slang, jigar is used as an adjective for any object which is desirable, especially women. In the Zulu language, the word for liver (isibindi) is the same as the word for courage.
The legend of Liver-Eating Johnson says that he would cut out and eat the liver of each man killed after dinner.
In the motion picture The Message, Hind bint Utbah is implied or portrayed eating the liver of Hamza ibn ‘Abd al-Muttalib during the Battle of Uhud. Although there are narrations that suggest that Hind did "taste", rather than eat, the liver of Hamza, the authenticity of these narrations have to be questioned.
Food
The liver of mammals, fowl, and fish are commonly eaten as food by humans. Domestic pig, ox, lamb, calf, chicken, and goose livers are widely available from butchers and supermarkets.Liver can be baked, boiled, broiled, fried, stir-fried, or eaten raw (asbeh nayeh or sawda naye in Lebanese cuisine, liver sashimi). In many preparations, pieces of liver are combined with pieces of meat or kidneys, like in the various forms of Middle Eastern mixed grill (e.g. meurav Yerushalmi). Liver is often made into spreads. Well-known examples include liver pâté, foie gras, chopped liver, and leverpastej. Liver sausages such as Braunschweiger and liverwurst are also a valued meal. Liver sausages may also be used as spreads. A traditional South African delicacy, namely Skilpadjies, is made of minced lamb's liver wrapped in netvet (caul fat), and grilled over an open fire.
Animal livers are rich in iron and vitamin A, and cod liver oil is commonly used as a dietary supplement. Traditionally, some fish livers were valued as food, especially the stingray liver. It was used to prepare delicacies, such as poached skate liver on toast in England, as well as the beignets de foie de raie and foie de raie en croute in French cuisine.[30]
Other animals
The liver is found in all vertebrates, and is typically the largest visceral (internal) organ. Its form varies considerably in different species, and is largely determined by the shape and arrangement of the surrounding organs. Nonetheless, in most species it is divided into right and left lobes; exceptions to this general rule include snakes, where the shape of the body necessitates a simple cigar-like form. The internal structure of the liver is broadly similar in all vertebrates.[31]
An organ sometimes referred to as a liver is found associated with the digestive tract of the primitive chordate Amphioxus. However, this is an enzyme secreting gland, not a metabolic organ, and it is unclear how truly homologous it is to the vertebrate liver.[31]