From Wikipedia, the free encyclopedia
Chlorofluorocarbons (
CFCs) are fully halogenated paraffin hydrocarbons that contain only
carbon (С),
chlorine (Cl), and
fluorine (F), produced as
volatile derivative of
methane,
ethane, and
propane. They are also commonly known by the
DuPont brand name
Freon. The most common representative is
dichlorodifluoromethane (R-12 or Freon-12). Many CFCs have been widely used as
refrigerants, propellants (in aerosol applications), and solvents. Because CFCs contribute to
ozone depletion in the upper
atmosphere, the manufacture of such compounds has been phased out under the
Montreal Protocol, and they are being replaced with other products such as
hydrofluorocarbons (HFCs)
[1] (e.g.,
R-410A) and R-134a.
[2][3]
Structure, properties and production
As in simpler
alkanes, carbon in the CFCs bonds with
tetrahedral
symmetry. Because the fluorine and chlorine atoms differ greatly in
size and effective charge from hydrogen and from each other, the
methane-derived CFCs deviate from perfect tetrahedral symmetry.
[4]
The physical properties of CFCs and HCFCs are tunable by changes in the number and identity of the
halogen
atoms. In general, they are volatile but less so than their parent
alkanes. The decreased volatility is attributed to the molecular
polarity induced by the
halides, which induces intermolecular interactions. Thus, methane boils at −161 °C whereas the fluoromethanes boil between −51.7 (CF
2H
2) and −128 °C (CF
4).
The CFCs have still higher boiling points because the chloride is even
more polarizable than fluoride. Because of their polarity, the CFCs are
useful solvents, and their boiling points make them suitable as
refrigerants. The CFCs are far less flammable than methane, in part
because they contain fewer C-H bonds and in part because, in the case of
the chlorides and bromides, the released halides quench the free
radicals that sustain flames.
The densities of CFCs are higher than their corresponding alkanes. In
general, the density of these compounds correlates with the number of
chlorides.
CFCs and HCFCs are usually produced by halogen exchange starting from
chlorinated methanes and ethanes. Illustrative is the synthesis of
chlorodifluoromethane from
chloroform:
- HCCl3 + 2 HF → HCF2Cl + 2 HCl
The brominated derivatives are generated by free-radical reactions of
the chlorofluorocarbons, replacing C-H bonds with C-Br bonds. The
production of the
anesthetic 2-bromo-2-chloro-1,1,1-trifluoroethane ("halothane") is illustrative:
- CF3CH2Cl + Br2 → CF3CHBrCl + HBr
Reactions
The most important reaction
[citation needed] of the CFCs is the
photo-induced scission of a C-Cl bond:
- CCl3F → CCl2F. + Cl.
The chlorine atom, written often as Cl
., behaves very differently from the chlorine molecule (Cl
2). The radical Cl
. is long-lived in the upper atmosphere, where it catalyzes the conversion of ozone into O
2. Ozone absorbs UV-B radiation, so its depletion allows more of this high energy radiation to reach the Earth's surface.
Bromine atoms are even more efficient catalysts; hence brominated CFCs are also regulated.
Applications
CFCs
and HCFCs are used in a variety of applications because of their low
toxicity, reactivity and flammability. Every permutation of fluorine,
chlorine and hydrogen based on methane and ethane has been examined and
most have been commercialized. Furthermore, many examples are known for
higher numbers of carbon as well as related compounds containing
bromine. Uses include
refrigerants,
blowing agents, propellants in medicinal applications and degreasing solvents.
Billions of kilograms of chlorodifluoromethane are produced annually as precursor to
tetrafluoroethylene, the monomer that is converted into
Teflon.
[5]
Classes of compounds, nomenclature
- Chlorofluorocarbons (CFCs): when derived from methane and ethane these compounds have the formulae CClmF4−m and C2ClmF6−m, where m is nonzero.
- Hydro-chlorofluorocarbons (HCFCs): when derived from methane and ethane these compounds have the formula CClmFnH4−m−n and C2ClxFyH6−x−y, where m, n, x, and y are nonzero.
- and bromofluorocarbons have formulae similar to the CFCs and HCFCs but also include bromine.
- Hydrofluorocarbons (HFCs): when derived from methane, ethane, propane, and butane, these compounds have the respective formulae CFmH4−m, C2FmH6−m, C3FmH8−m, and C4FmH10−m, where m is nonzero.
Numbering system
A special numbering system is used for fluorinated alkanes, prefixed
with Freon-, R-, CFC- and HCFC-, where the rightmost value indicates the
number of fluorine atoms, the next value to the left is the number of
hydrogen atoms
plus 1, and the next value to the left is the number of carbon atoms
less one (zeroes are not stated), and the remaining atoms are
chlorine.
Freon-12, for example, indicates a methane derivative (only two
numbers) containing two fluorine atoms (the second 2) and no hydrogen
(1-1=0). It is therefore CCl
2F
2.
Another, easier equation that can be applied to get the correct
molecular formula of the CFC/R/Freon class compounds is this to take the
numbering and add 90 to it. The resulting value will give the number of
carbons as the first numeral, the second numeral gives the number of
hydrogen atoms, and the third numeral gives the number of fluorine
atoms. The rest of the unaccounted carbon bonds are occupied by chlorine
atoms. The value of this equation is always a three figure number. An
easy example is that of CFC-12, which gives: 90+12=102 -> 1 carbon, 0
hydrogens, 2 fluorine atoms, and hence 2 chlorine atoms resulting in
CCl
2F
2. The main advantage of this method of
deducing the molecular composition in comparison with the method
described in the paragraph above is that it gives the number of carbon
atoms of the molecule.
History
Carbon tetrachloride (CCl
4) was used in fire extinguishers and glass "anti-fire grenades" from the late nineteenth century until around the end of
World War II. Experimentation with chloroalkanes for fire suppression on military
aircraft began at least as early as the 1920s.
Freon is a trade name for a group of CFCs which are used primarily as
refrigerants, but also have uses in fire-fighting and as propellants in
aerosol cans. Bromomethane is widely used as a fumigant. Dichloromethane is a versatile industrial solvent.
The Belgian scientist
Frédéric Swarts
pioneered the synthesis of CFCs in the 1890s. He developed an effective
exchange agent to replace chloride in carbon tetrachloride with
fluoride to synthesize CFC-11 (CCl
3F) and CFC-12 (CCl
2F
2).
In the late 1920s,
Thomas Midgley, Jr. improved the process of synthesis and led the effort to use CFC as refrigerant to replace
ammonia (NH
3),
chloromethane (CH
3Cl), and
sulfur dioxide (SO
2), which are toxic but were in common use. In searching for a new refrigerant, requirements for the compound were: low
boiling point, low toxicity, and to be generally non-reactive. In a demonstration for the
American Chemical Society, Midgley flamboyantly demonstrated all these properties by inhaling a breath of the gas and using it to blow out a candle
[6] in 1930.
[7][8]
Commercial development and use
During
World War II,
various chloroalkanes were in standard use in military aircraft,
although these early halons suffered from excessive toxicity.
Nevertheless, after the war they slowly became more common in civil
aviation as well. In the 1960s, fluoroalkanes and bromofluoroalkanes
became available and were quickly recognized as being highly effective
fire-fighting materials. Much early research with
Halon 1301 was conducted under the auspices of the US Armed Forces, while
Halon 1211
was, initially, mainly developed in the UK. By the late 1960s they were
standard in many applications where water and dry-powder extinguishers
posed a threat of damage to the protected property, including computer
rooms, telecommunications switches, laboratories, museums and art
collections. Beginning with
warships,
in the 1970s, bromofluoroalkanes also progressively came to be
associated with rapid knockdown of severe fires in confined spaces with
minimal risk to personnel.
By the early 1980s, bromofluoroalkanes were in common use on
aircraft, ships, and large vehicles as well as in computer facilities
and galleries. However, concern was beginning to be expressed about the
impact of chloroalkanes and bromoalkanes on the
ozone layer. The
Vienna Convention for the Protection of the Ozone Layer
did not cover bromofluoroalkanes as it was thought, at the time, that
emergency discharge of extinguishing systems was too small in volume to
produce a significant impact, and too important to human safety for
restriction.
Regulation
Since the late 1970s, the use of CFCs has been heavily regulated because of their destructive effects on the
ozone layer. After the development of his
electron capture detector,
James Lovelock was the first to detect the widespread presence of CFCs in the air, finding a
mole fraction of 60
ppt of CFC-11 over
Ireland.
In a self-funded research expedition ending in 1973, Lovelock went on
to measure CFC-11 in both the Arctic and Antarctic, finding the presence
of the gas in each of 50 air samples collected, and concluding that
CFCs are not hazardous to the environment. The experiment did however
provide the first useful data on the presence of CFCs in the atmosphere.
The damage caused by CFCs was discovered by
Sherry Rowland and
Mario Molina
who, after hearing a lecture on the subject of Lovelock's work,
embarked on research resulting in the first publication suggesting the
connection in 1974. It turns out that one of CFCs' most attractive
features—their low reactivity— is key to their most destructive effects.
CFCs' lack of reactivity gives them a lifespan that can exceed 100
years, giving them time to diffuse into the upper
stratosphere.
[9] Once in the stratosphere, the sun's
ultraviolet radiation is strong enough to cause the
homolytic cleavage of the C-Cl bond.
By 1987, in response to a dramatic seasonal depletion of the ozone layer over
Antarctica, diplomats in
Montreal forged a treaty, the
Montreal Protocol, which called for drastic reductions in the production of CFCs. On 2 March 1989, 12
European Community nations agreed to ban the production of all CFCs by the end of the century. In 1990, diplomats met in
London
and voted to significantly strengthen the Montreal Protocol by calling
for a complete elimination of CFCs by the year 2000. By the year 2010,
CFCs should have been completely eliminated from developing countries as
well.
Ozone-depleting gas trends
Because the only CFCs available to countries adhering to the treaty
is from recycling, their prices have increased considerably. A worldwide
end to production should also terminate the smuggling of this material.
However, there are current CFC smuggling issues, as recognized by the
United Nations Environmental Programme
(UNEP) in a 2006 report titled "Illegal Trade in Ozone Depleting
Substances". UNEP estimates that between 16,000–38,000 tonnes of CFCs
passed through the black market in the mid-1990s. The report estimated
between 7,000 and 14,000 tonnes of CFCs are smuggled annually into
developing countries. Asian countries are those with the most smuggling;
as of 2007, China, India and South Korea were found to account for
around 70% of global CFC production,
[10] South Korea later to ban CFC production in 2010.
[11]
Possible reasons for continued CFC smuggling were also examined: the
report noted that many banned CFC producing products have long lifespans
and continue to operate. The cost of replacing the equipment of these
items is sometimes cheaper than outfitting them with a more
ozone-friendly appliance. Additionally, CFC smuggling is not considered a
significant issue, so the perceived penalties for smuggling are low. In
2018 public attention was drawn to the issue, that at an unknown place
in east Asia an estimated amount of 13.000 metric tons anually of CFCs
have been produced since about 2012 in violation of the protocol.
[12][13] While the eventual phaseout of CFCs is likely, efforts are being taken to stem these current non-compliance problems.
By the time of the
Montreal Protocol,
it was realised that deliberate and accidental discharges during system
tests and maintenance accounted for substantially larger volumes than
emergency discharges, and consequently halons were brought into the
treaty, albeit with many exceptions.
Regulatory gap
While
the production and consumption of CFCs are regulated under the Montreal
Protocol, emissions from existing banks of CFCs are not regulated under
the agreement. In 2002, there were an estimated 5,791 kilotons of CFCs
in existing products such as refrigerators, air conditioners, aerosol
cans and others.
[14]
Approximately one-third of these CFCs are projected to be emitted over
the next decade if action is not taken, posing a threat to both the
ozone layer and the climate.
[15] A proportion of these CFCs can be safely captured and destroyed.
Regulation and DuPont
In
1978 the United States banned the use of CFCs such as Freon in aerosol
cans, the beginning of a long series of regulatory actions against their
use. The critical DuPont manufacturing patent for Freon ("Process for
Fluorinating Halohydrocarbons", U.S. Patent #3258500) was set to expire
in 1979. In conjunction with other industrial peers DuPont formed a
lobbying group, the "Alliance for Responsible CFC Policy," to combat
regulations of ozone-depleting compounds.
[16] In 1986 DuPont, with new patents in hand, reversed its previous stance and publicly condemned CFCs.
[17] DuPont representatives appeared before the
Montreal Protocol urging that CFCs be banned worldwide and stated that their new HCFCs would meet the worldwide demand for refrigerants.
[17]
Phasing-out of CFCs
Use
of certain chloroalkanes as solvents for large scale application, such
as dry cleaning, have been phased out, for example, by the
IPPC directive on
greenhouse gases in 1994 and by the
volatile organic compounds (VOC) directive of the
EU in 1997. Permitted chlorofluoroalkane uses are medicinal only.
Bromofluoroalkanes have been largely phased out and the possession of
equipment for their use is prohibited in some countries like the
Netherlands and Belgium, from 1 January 2004, based on the
Montreal Protocol and guidelines of the European Union.
Production of new stocks ceased in most (probably all) countries in 1994.
[citation needed]
However many countries still require aircraft to be fitted with halon
fire suppression systems because no safe and completely satisfactory
alternative has been discovered for this application. There are also a
few other, highly specialized uses. These programs recycle halon through
"halon banks" coordinated by the Halon Recycling Corporation
[18] to ensure that discharge to the atmosphere occurs only in a genuine emergency and to conserve remaining stocks.
The interim replacements for CFCs are hydrochlorofluorocarbons
(HCFCs), which deplete stratospheric ozone, but to a much lesser extent
than CFCs.
[19] Ultimately,
hydrofluorocarbons (HFCs) will replace HCFCs. Unlike CFCs and HCFCs, HFCs have an ozone depletion potential (ODP) of 0.
[20]
DuPont began producing hydrofluorocarbons as alternatives to Freon in
the 1980s. These included Suva refrigerants and Dymel propellants.
[21]
Natural refrigerants are climate friendly solutions that are enjoying
increasing support from large companies and governments interested in
reducing global warming emissions from refrigeration and air
conditioning. Hydrofluorocarbons are included in the
Kyoto Protocol because of their very high
Global Warming Potential and are facing calls to be regulated under the
Montreal Protocol[dubious – discuss][22] due to the recognition of halocarbon contributions to climate change.
[23]
On 21 September 2007, approximately 200 countries agreed to
accelerate the elimination of hydrochlorofluorocarbons entirely by 2020
in a
United Nations-sponsored
Montreal summit. Developing nations were given until 2030. Many nations, such as the
United States and
China, who had previously
resisted such efforts, agreed with the accelerated phase out schedule.
[24]
Development of alternatives for CFCs
Work on alternatives for chlorofluorocarbons in refrigerants began in the late 1970s after the first warnings of damage to
stratospheric ozone were published.
The hydrochlorofluorocarbons (HCFCs) are less stable in the lower
atmosphere, enabling them to break down before reaching the ozone layer.
Nevertheless, a significant fraction of the HCFCs do break down in the
stratosphere
and they have contributed to more chlorine buildup there than
originally predicted. Later alternatives lacking the chlorine, the
hydrofluorocarbons (HFCs) have an even shorter lifetimes in the lower
atmosphere.
[19] One of these compounds,
HFC-134a,
is now used in place of CFC-12 in automobile air conditioners.
Hydrocarbon refrigerants (a propane/isobutane blend) are also used
extensively in mobile air conditioning systems in Australia, the USA and
many other countries, as they have excellent thermodynamic properties
and perform particularly well in high ambient temperatures.
One of the natural refrigerants (along with ammonia and carbon
dioxide), hydrocarbons have negligible environmental impacts and are
also used worldwide in domestic and commercial refrigeration
applications, and are becoming available in new split system air
conditioners.
[25] Various other solvents and methods have replaced the use of CFCs in laboratory analytics.
[26]
In
Metered-dose inhalers (MDI), a non-ozone effecting substitute was developed as a propellant, known as "
hydrofluoroalkane."
[27]
Environmental impacts
As previously discussed, CFCs were phased out via the
Montreal Protocol
due to their part in ozone depletion. However, the atmospheric impacts
of CFCs are not limited to its role as an active ozone reducer. This
anthropogenic compound is also a
greenhouse gas, with a much higher potential to enhance the greenhouse effect than CO
2.
Infrared absorption bands trap heat from escaping earth's atmosphere.
In the case of CFCs, the strongest of these bands are located in the
spectral region 7.8–15.3
µm [28] – referred to as an atmospheric window due to the relative transparency of the atmosphere within this region.
[29]
The strength of CFC bands and the unique susceptibility of the
atmosphere, at which the compound absorbs and emits radiation, are two
factors that contribute to CFCs' "super" greenhouse effect.
[30] Another such factor is the low concentration of the compound. Because CO
2 is close to saturation with high concentrations,
[clarification needed]
it takes more of the substance to enhance the greenhouse effect.
Conversely, the low concentration of CFCs allow their effects to
increase linearly with mass.
[30]
Tracer of ocean circulation
Because
the time history of CFC concentrations in the atmosphere is relatively
well known, they have provided an important constraint on ocean
circulation. CFCs dissolve in seawater at the ocean surface and are
subsequently transported into the ocean interior. Because CFCs are
inert, their concentration in the ocean interior reflects simply the
convolution of their atmospheric time evolution and ocean circulation
and mixing.
CFC and SF6 tracer-derived age of ocean water
Chlorofluorocarbons
(CFCs) are anthropogenic compounds that have been released into the
atmosphere since the 1930s in various applications such as in
air-conditioning, refrigeration, blowing agents in foams, insulations
and packing materials, propellants in aerosol cans, and as solvents.
[31] The entry of CFCs into the ocean makes them extremely useful as
transient tracers to estimate rates and pathways of ocean circulation
and mixing processes.
[32]
However, due to production restrictions of CFCs in the 1980s,
atmospheric concentrations of CFC-11 and CFC-12 has stopped increasing,
and the CFC-11 to CFC-12 ratio in the atmosphere have been steadily
decreasing, making water dating of water masses more problematic.
[32] Incidentally, production and release of sulfur hexafluoride (SF
6) have rapidly increased in the atmosphere since the 1970s.
[32] Similar to CFCs, SF
6 is also an inert gas and is not affected by oceanic chemical or biological activities.
[33] Thus, using CFCs in concert with SF
6 as a tracer resolves the water dating issues due to decreased CFC concentrations.
Using CFCs or SF
6 as a tracer of ocean circulation allows
for the derivation of rates for ocean processes due to the
time-dependent source function. The elapsed time since a subsurface
water mass was last in contact with the atmosphere is the tracer-derived
age.
[34]
Estimates of age can be derived based on the partial pressure of an
individual compound and the ratio of the partial pressure of CFCs to
each other (or SF
6).
[34]
Partial pressure and ratio dating techniques
The age of a water parcel can be estimated by the CFC partial pressure (pCFC) age or SF
6 partial pressure (pSF
6) age. The pCFC age of a water sample is defined as:
![{\displaystyle pCFC={\frac {[CFC]}{F(T,S)}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d2662166456bba71b0bd9991a30d238d64b6e23e)
where [CFC] is the measured CFC concentration (pmol kg
−1) and F is the solubility of CFC gas in seawater as a function of temperature and salinity.
[35] The CFC partial pressure is expressed in units of 10–12 atmospheres or parts-per-trillion (ppt).
[36] The solubility measurements of CFC-11 and CFC-12 have been previously measured by Warner and Weiss
[36] Additionally, the solubility measurement of CFC-113 was measured by Bu and Warner
[37] and SF
6 by Wanninkhof et al.
[38] and Bullister et al.
[39] Theses authors mentioned above have expressed the solubility, F, at a total pressure of 1 atm as:
![{\displaystyle \ln F=a_{1}+a_{2}\left({\frac {100}{T}}\right)+a_{3}\ln \left({\frac {T}{100}}\right)+a_{4}\left({\frac {T}{100}}\right)^{2}+S\left[b_{1}+b_{2}\left({\frac {T}{100}}\right)+b_{3}\left({\frac {T}{100}}\right)\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2f818e2e34a54f1e5e60d3afb971d967d891a331)
where F = solubility expressed in either mol l
−1 or mol kg
−1 atm
−1, T = absolute temperature, S = salinity in parts per thousand (ppt), a
1, a
2, a
3, b
1, b
2, and b
3 are constants to be determined from the least squares fit to the solubility measurements.
[37] This equation is derived from the integrated
Van 't Hoff equation and the logarithmic Setchenow salinity dependence.
[37]
It can be noted that the solubility of CFCs increase with decreasing temperature at approximately 1% per degree Celsius.
[34]
Once the partial pressure of the CFC (or SF
6) is derived, it is then compared to the atmospheric time histories for CFC-11, CFC-12, or SF
6
in which the pCFC directly corresponds to the year with the same. The
difference between the corresponding date and the collection date of the
seawater sample is the average age for the water parcel.
[34] The age of a parcel of water can also be calculated using the ratio of two CFC partial pressures or the ratio of the SF
6 partial pressure to a CFC partial pressure.
[34]
Safety
According
to their material safety data sheets, CFCs and HCFCs are colorless,
volatile, toxic liquids and gases with a faintly sweet ethereal odor.
Overexposure at concentrations of 11% or more may cause dizziness, loss
of concentration, central nervous system depression and/or
cardiac arrhythmia. Vapors displace air and can cause asphyxiation in confined spaces.
Although non-flammable, their combustion products include hydrofluoric
acid, and related species.
[40] Normal occupational exposure is rated at 0.07% and does not pose any serious health risks.
[41]











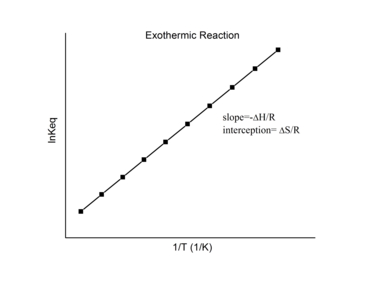






![{\displaystyle {\begin{aligned}\Delta H_{1}&=-R\times {\text{slope}}_{1},&\Delta S_{1}&=R\times {\text{intercept}}_{1};\\[5pt]\Delta H_{2}&=-R\times {\text{slope}}_{2},&\Delta S_{2}&=R\times {\text{intercept}}_{2}.\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2a6c1cf504bbb52519d024ecbad81f7a50dee58f)







![{\displaystyle pCFC={\frac {[CFC]}{F(T,S)}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d2662166456bba71b0bd9991a30d238d64b6e23e)
