Some philosophers, such as Jeremy Bentham, Baruch Spinoza, and Descartes, have hypothesized that the feelings of pain (or suffering) and pleasure are part of a continuum.
There is strong evidence of biological connections between the neurochemical pathways used for the perception of both pain and pleasure, as well as other psychological rewards.
Perception of pain
Sensory input system
From a stimulus-response perspective, the perception of physical pain starts with the nociceptors,
a type of physiological receptor that transmits neural signals to the
brain when activated. These receptors are commonly found in the skin,
membranes, deep fascias, mucosa, connective tissues of visceral organs,
ligaments and articular capsules, muscles, tendons, periosteum, and
arterial vessels.
Once stimuli are received, the various afferent action potentials are
triggered and pass along various fibers and axons of these nociceptive
nerve cells into the dorsal horn of the spinal cord through the dorsal
roots. A neuroanatomical review of the pain pathway, "Afferent pain
pathways" by Almeida, describes various specific nociceptive pathways of
the spinal cord: spinothalamic tract, spinoreticular tract, spinomesencephalic tract, spinoparabrachial tract, spinohypothalamic tract, spinocervical tract, postsynaptic pathway of the spinal column.
Neural coding and modulation
Activity
in many parts of the brain is associated with pain perception. Some of
the known parts for the ascending pathway include the thalamus, hypothalamus, midbrain, lentiform nucleus, somatosensory cortices, insular, prefrontal, anterior and parietal cingulum.
Then, there are also the descending pathways for the modulation of
pain sensation. One of the brainstem regions responsible for this is the
periaqueductal gray
of the midbrain, which both relieves pain by behavior as well as
inhibits the activity of the nociceptive neurons in the dorsal horn of
the spinal cord. Other brainstem sites, such as the parabrachial
nucleus, the dorsal raphe, locus coeruleus,
and the medullary reticular formation also mediate pain relief and use
many different neurotransmitters to either facilitate or inhibit
activity of the neurons in the dorsal horn. These neurotransmitters
include noradrenaline, serotonin, dopamine, histamine, and acetylcholine.
Perception of pleasure
Pleasure
can be considered from many different perspectives, from physiological
(such as the hedonic hotspots that are activated during the experience)
to psychological (such as the study of behavioral responses towards
reward). Pleasure has also often been compared to, or even defined by
many neuroscientists as, a form of alleviation of pain.
Neural coding and modulation
Pleasure
has been studied in the systems of taste, olfaction, auditory
(musical), visual (art), and sexual activity. Well known hedonic
hotspots involved in the processing of pleasure include the nucleus accumbens, posterior ventral pallidum, amygdala, other cortical and subcortical regions.
The prefrontal and limbic regions of the neocortex, particularly the orbitofrontal region of the prefrontal cortex, anterior cingulate cortex, and the insular cortex have all been suggested to be pleasure causing substrates in the brain.
Psychology of pain and pleasure (reward-punishment system)
One
approach to evaluating the relationship between pain and pleasure is to
consider these two systems as a reward-punishment based system. When
pleasure is perceived, one associates it with reward. When pain is
perceived, one associates with punishment. Evolutionarily, this makes
sense, because often, actions that result in pleasure or chemicals that
induce pleasure work towards restoring homeostasis in the body. For
example, when the body is hungry, the pleasure of rewarding food to
one-self restores the body back to a balanced state of replenished
energy. Like so, this can also be applied to pain, because the ability
to perceive pain enhances both avoidance and defensive mechanisms that
were, and still are, necessary for survival.
Opioid and dopamine systems in pain and pleasure
The neural systems to be explored when trying to look for a neurochemical relationship between pain and pleasure are the opioid and dopamine
systems. The opioid system is responsible for the actual experience of
the sensation, whereas the dopamine system is responsible for the
anticipation or expectation of the experience. Opioids work in the
modulation of pleasure or pain relief by either blocking
neurotransmitter release or by hyperpolarizing neurons by opening up a
potassium channel which effectively temporarily blocks the neuron.
Pain and pleasure on a continuum
Arguments for pain and pleasure on a continuum
It has been suggested as early as 4th century BC that pain and pleasure occurs on a continuum. Aristotle claims this antagonistic relationship in his Rhetoric:
- "We may lay it down that Pleasure is a movement, a movement by which the soul as a whole is consciously brought into its normal state of being; and that Pain is the opposite."
He describes pain and pleasure very much like a push-pull concept;
human beings will move towards something that causes pleasure and will
move away from something that causes pain.
Common neuroanatomy
On
an anatomical level, it can be shown the source for the modulation of
both pain and pleasure originates from neurons in the same locations,
including the amygdala, the pallidum, and the nucleus accumbens.
Not only have Leknes and Tracey, two leading neuroscientists in the
study of pain and pleasure, concluded that pain and reward processing
involve many of the same regions of the brain, but also that the
functional relationship lies in that pain decreases pleasure and rewards
increase analgesia, which is the relief from pain.
Arguments against pain and pleasure on a continuum
Asymmetry between pain and pleasure
Thomas
Szasz, the late Professor of Psychiatry Emeritus at the State
University of New York Health Science Center in Syracuse, New York,
explored how pain and pleasure are not opposites ends of a spectrum in
his 1957 book, "Pain and Pleasure -a study of bodily feelings".
Szasz notes that although we often refer to pain and pleasure as
opposites in such a way, that this is incorrect; we have receptors for
pain, but none in the same way for pleasure; and so it makes sense to
ask "where is the pain?" but not "where is the pleasure?". With this
vantage point established, the author delves into the topics of
metaphorical pain and of legitimacy, of power relations, and of
communications, and of myriad others.
Evolutionary hypotheses for the relationship between pain and pleasure
Whether
or not pain and pleasure are indeed on a continuum, it still remains
scientifically supported that parts of the neural pathways for the two
perceptions overlap. There is also scientific evidence that one may have
opposing effects on the other. So why would it be evolutionarily
advantageous to human beings to develop a relationship between the two
perceptions at all?
South African neuroscientists presented evidence that there was a
physiological link on a continuum between pain and pleasure in 1980.
First, the Neuroscientists, Gillman and Lichtigfeld demonstrated that
there were two endogenous endorphin systems, one pain producing and the
other pain relieving.
A short time later they showed that these two systems might also be
involved in addiction, which is initially pursued, presumably for the
pleasure generating or pain relieving actions of the addictive
substance. Soon after they provided evidence that the endorphins system was involved in sexual pleasure.
Dr. Kringelbach suggests that this relationship between pain and
pleasure would be evolutionarily efficient, because it was necessary to
know whether or not to avoid or approach something for survival.
According to Dr. Norman Doidge,
the brain is limited in the sense that it tends to focus on the most
used pathways. Therefore, having a common pathway for pain and pleasure
could have simplified the way in which human beings have interacted with
the environment (Dr. Morten Kringelbach, personal communication, October 24, 2011).
Leknes and Tracey offer two theoretical perspectives to why a relationship could be evolutionarily advantageous.
Opponent process theory
The opponent-process theory
is a model that views two components as being pairs that are opposite
to each other, such that if one component is experienced, the other
component will be repressed. Therefore, an increase in pain should bring
about a decrease in pleasure, and a decrease in pain should bring about
an increase in pleasure or pain relief. This simple model serves the
purpose of explaining the evolutionarily significant role of homeostasis
in this relationship. This is evident since both seeking pleasure and
avoiding pain are important for survival. Leknes and Tracey provide an
example:
- "In the face of a large food reward, which can only be obtained at the cost of a small amount of pain, for instance, it would be beneficial if the pleasurable food reduced pain unpleasantness."
They then suggest that perhaps a common currency for which human
beings determine the importance of the motivation for each perception
can allow them to be weighed against each other in order to make a
decision best for survival.
Motivation-decision model
The Motivation-Decision Model, suggested by Fields,
is centered around the concept that decision processes are driven by
motivations of highest priority. The model predicts that in the case
that there is anything more important than pain for survival will cause
the human body to mediate pain by activating the descending pain
modulation system described earlier.
Thus, it is suggested that human beings have developed the unconscious
ability to endure pain or sometimes, even relieve pain if it can be
more important for survival to gain a larger reward. It may have been
more advantageous to link the pain and pleasure perceptions together to
be able to reduce pain to gain a reward necessary for fitness, such as
childbirth. Like the opponent-process theory, if the body can induce
pleasure or pain relief to decrease the effect of pain, it would allow
human beings to be able to make the best evolutionary decisions for
survival.
Clinical applications
Related diseases
The following neurological and/or mental diseases have been linked to forms of pain or anhedonia: schizophrenia, depression, addiction, cluster headache, chronic pain.
Animal trials
A great deal of what is known about pain and pleasure today primarily comes from studies conducted with rats and primates.
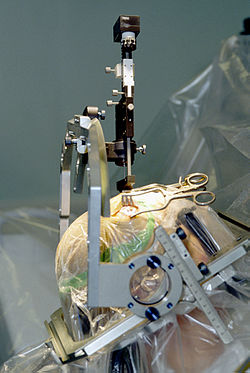
Insertion of electrode during Deep Brain Stimulation surgery using a stereotactic frame
Deep brain stimulation
Deep brain stimulation
involves the electrical stimulation of deep brain structures by
electrodes implanted into the brain. The effects of this neurosurgery
has been studied in patients with Parkinson's disease, tremors, dystonia, epilepsy, depression, obsessive-compulsive disorder, Tourette's syndrome, cluster headache and chronic pain.
A fine electrode is inserted into the targeted area of the brain and
secured to the skull. This is attached to a pulse generator which is
implanted elsewhere on the body under the skin. The surgeon then turns
the frequency of the electrode to the voltage and frequency desired.
Deep brain stimulation has been shown in several studies to both induce
pleasure or even addiction as well as ameliorate pain. For chronic pain,
lower frequencies (about 5–50 Hz) have produced analgesic effects,
whereas higher frequencies (about 120–180 Hz) have alleviated or stopped
pyramidal tremors in Parkinson's patients.
There is still further research necessary into how and why
exactly DBS works. However, by understanding the relationship between
pleasure and pain, procedures like these can be used to treat patients
suffering from a high intensity or longevity of pain. So far, DBS has
been recognized as a treatment for Parkinson's disease, tremors, and dystonia by the Food and Drug Administration (FDA).