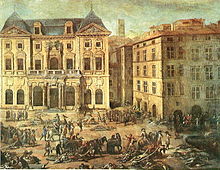| Infectious disease | |
|---|---|
 | |
| A false-colored electron micrograph shows a malaria sporozoite migrating through the midgut epithelium of a rat. | |
| Specialty | Infectious disease |
Infection is the invasion of an organism's body tissues by disease-causing agents, their multiplication, and the reaction of host tissues to the infectious agents and the toxins they produce. Infectious disease, also known as transmissible disease or communicable disease, is illness resulting from an infection.
Infections are caused by infectious agents including viruses, viroids, prions, bacteria, nematodes such as parasitic roundworms and pinworms, arthropods such as ticks, mites, fleas, and lice, fungi such as ringworm, and other macroparasites such as tapeworms and other helminths.
Hosts can fight infections using their immune system. Mammalian hosts react to infections with an innate response, often involving inflammation, followed by an adaptive response.
Specific medications used to treat infections include antibiotics, antivirals, antifungals, antiprotozoals, and antihelminthics. Infectious diseases resulted in 9.2 million deaths in 2013 (about 17% of all deaths). The branch of medicine that focuses on infections is referred to as infectious disease.
Classification
Subclinical versus clinical (latent versus apparent)
Symptomatic infections are apparent and clinical, whereas an infection that is active but does not produce noticeable symptoms may be called inapparent, silent, subclinical, or occult. An infection that is inactive or dormant is called a latent infection. An example of a latent bacterial infection is latent tuberculosis. Some viral infections can also be latent, examples of latent viral infections are any of those from the Herpesviridae family.
The word infection can denote any presence of a particular pathogen at all (no matter how little) but also is often used in a sense implying a clinically apparent infection (in other words, a case of infectious disease). This fact occasionally creates some ambiguity or prompts some usage discussion. To get around the usage annoyance, it is common for health professionals to speak of colonization (rather than infection) when they mean that some of the pathogens are present but that no clinically apparent infection (no disease) is present.
A short-term infection is an acute infection. A long-term infection is a chronic
infection. Infections can be further classified by causative agent
(bacterial, viral, fungal, parasitic), and by the presence or absence of
systemic symptoms (sepsis).
Primary versus opportunistic
Among the many varieties of microorganisms, relatively few cause disease in otherwise healthy individuals. Infectious disease results from the interplay between those few pathogens
and the defenses of the hosts they infect. The appearance and severity
of disease resulting from any pathogen, depends upon the ability of that
pathogen to damage the host as well as the ability of the host to
resist the pathogen. However a host's immune system can also cause
damage to the host itself in an attempt to control the infection.
Clinicians therefore classify infectious microorganisms or microbes
according to the status of host defenses - either as primary pathogens or as opportunistic pathogens:
- Primary pathogens
- Primary pathogens cause disease as a result of their presence or activity within the normal, healthy host, and their intrinsic virulence (the severity of the disease they cause) is, in part, a necessary consequence of their need to reproduce and spread. Many of the most common primary pathogens of humans only infect humans, however many serious diseases are caused by organisms acquired from the environment or that infect non-human hosts.
- Opportunistic pathogens
- Opportunistic pathogens can cause an infectious disease in a host with depressed resistance (immunodeficiency) or if they have unusual access to the inside of the body (for example, via trauma). Opportunistic infection may be caused by microbes ordinarily in contact with the host, such as pathogenic bacteria or fungi in the gastrointestinal or the upper respiratory tract, and they may also result from (otherwise innocuous) microbes acquired from other hosts (as in Clostridium difficile colitis) or from the environment as a result of traumatic introduction (as in surgical wound infections or compound fractures). An opportunistic disease requires impairment of host defenses, which may occur as a result of genetic defects (such as Chronic granulomatous disease), exposure to antimicrobial drugs or immunosuppressive chemicals (as might occur following poisoning or cancer chemotherapy), exposure to ionizing radiation, or as a result of an infectious disease with immunosuppressive activity (such as with measles, malaria or HIV disease). Primary pathogens may also cause more severe disease in a host with depressed resistance than would normally occur in an immunosufficient host.
- Primary infection versus secondary infection
- A primary infection is infection that is, or can practically be viewed as, the root cause of the current health problem. In contrast, a secondary infection is a sequela or complication of a root cause. For example, pulmonary tuberculosis is often a primary infection, but an infection that happened only because a burn or penetrating trauma (the root cause) allowed unusual access to deep tissues is a secondary infection. Primary pathogens often cause primary infection and also often cause secondary infection. Usually opportunistic infections are viewed as secondary infections (because immunodeficiency or injury was the predisposing factor).
Infectious or not
One way of proving that a given disease is "infectious", is to satisfy Koch's postulates (first proposed by Robert Koch), which demands that the infectious agent
be identified only in patients and not in healthy controls, and that
patients who contract the agent also develop the disease. These
postulates were first used in the discovery that Mycobacteria species cause tuberculosis.
Koch's postulates cannot be applied ethically for many human diseases
because they require experimental infection of a healthy individual with
a pathogen produced as a pure culture. Often, even clearly infectious
diseases do not meet the infectious criteria. For example, Treponema pallidum, the causative spirochete of syphilis, cannot be cultured in vitro – however the organism can be cultured in rabbit testes.
It is less clear that a pure culture comes from an animal source
serving as host than it is when derived from microbes derived from plate
culture.
Epidemiology is another important tool used to study disease in a population. For infectious diseases it helps to determine if a disease outbreak is sporadic (occasional occurrence), endemic (regular cases often occurring in a region), epidemic (an unusually high number of cases in a region), or pandemic (a global epidemic).
Contagiousness
Infectious diseases are sometimes called contagious disease when they are easily transmitted by contact with an ill person or their secretions (e.g., influenza).
Thus, a contagious disease is a subset of infectious disease that is
especially infective or easily transmitted. Other types of infectious/
By anatomic location
Infections can be classified by the anatomic location or organ system infected, including:
- Urinary tract infection
- Skin infection
- Respiratory tract infection
- Odontogenic infection (an infection that originates within a tooth or in the closely surrounding tissues)
- Vaginal infections
- Intra-amniotic infection
In addition, locations of inflammation where infection is the most common cause include pneumonia, meningitis and salpingitis.
Signs and symptoms
The symptoms of an infection depends on the type of disease. Some signs of infection affect the whole body generally, such as fatigue, loss of appetite, weight loss, fevers, night sweats, chills, aches and pains. Others are specific to individual body parts, such as skin rashes, coughing, or a runny nose.
In certain cases, infectious diseases may be asymptomatic
for much or even all of their course in a given host. In the latter
case, the disease may only be defined as a "disease" (which by
definition means an illness) in hosts who secondarily become ill after
contact with an asymptomatic carrier. An infection is not synonymous
with an infectious disease, as some infections do not cause illness in a
host.
Bacterial or viral
Bacterial
and viral infections can both cause the same kinds of symptoms. It can
be difficult to distinguish which is the cause of a specific infection. It's important to distinguish, because viral infections cannot be cured by antibiotics.
| Characteristic | Viral infection | Bacterial infection |
|---|---|---|
| Typical symptoms | In general, viral infections are systemic. This means they involve many different parts of the body or more than one body system at the same time; i.e. a runny nose, sinus congestion, cough, body aches etc. They can be local at times as in viral conjunctivitis or "pink eye" and herpes. Only a few viral infections are painful, like herpes. The pain of viral infections is often described as itchy or burning. | The classic symptoms of a bacterial infection are localized redness, heat, swelling and pain. One of the hallmarks of a bacterial infection is local pain, pain that is in a specific part of the body. For example, if a cut occurs and is infected with bacteria, pain occurs at the site of the infection. Bacterial throat pain is often characterized by more pain on one side of the throat. An ear infection is more likely to be diagnosed as bacterial if the pain occurs in only one ear. A cut that produces pus and milky-colored liquid is most likely infected. |
| Cause | Pathogenic viruses | Pathogenic bacteria |
Pathophysiology
There is a general chain of events that applies to infections.
The chain of events involves several steps—which include the infectious
agent, reservoir, entering a susceptible host, exit and transmission to
new hosts. Each of the links must be present in a chronological order
for an infection to develop. Understanding these steps helps health care
workers target the infection and prevent it from occurring in the first
place.
Colonization
Infection of an ingrown toenail; there is pus (yellow) and resultant inflammation (redness and swelling around the nail).
Infection begins when an organism successfully enters the body, grows
and multiplies. This is referred to as colonization. Most humans are
not easily infected. Those who are weak, sick, malnourished, have cancer
or are diabetic have increased susceptibility to chronic or persistent
infections. Individuals who have a suppressed immune system are particularly susceptible to opportunistic infections. Entrance to the host at host-pathogen interface, generally occurs through the mucosa in orifices like the oral cavity,
nose, eyes, genitalia, anus, or the microbe can enter through open
wounds. While a few organisms can grow at the initial site of entry,
many migrate and cause systemic infection in different organs. Some
pathogens grow within the host cells (intracellular) whereas others grow
freely in bodily fluids.
Wound
colonization refers to nonreplicating microorganisms within the wound,
while in infected wounds, replicating organisms exist and tissue is
injured. All multicellular organisms are colonized to some degree by extrinsic organisms, and the vast majority of these exist in either a mutualistic or commensal relationship with the host. An example of the former is the anaerobic bacteria species, which colonizes the mammalian colon, and an example of the latter are the various species of staphylococcus that exist on human skin.
Neither of these colonizations are considered infections. The
difference between an infection and a colonization is often only a
matter of circumstance. Non-pathogenic organisms can become pathogenic
given specific conditions, and even the most virulent organism requires certain circumstances to cause a compromising infection. Some colonizing bacteria, such as Corynebacteria sp. and viridans streptococci,
prevent the adhesion and colonization of pathogenic bacteria and thus
have a symbiotic relationship with the host, preventing infection and
speeding wound healing.
This image depicts the steps of pathogenic infection.
The variables involved in the outcome of a host becoming inoculated by a pathogen and the ultimate outcome include:
- the route of entry of the pathogen and the access to host regions that it gains
- the intrinsic virulence of the particular organism
- the quantity or load of the initial inoculant
- the immune status of the host being colonized
As an example, several staphylococcal species remain harmless on the skin, but, when present in a normally sterile space, such as in the capsule of a joint or the peritoneum, multiply without resistance and cause harm.
An interesting fact that gas chromatography–mass spectrometry, 16S ribosomal RNA analysis, omics,
and other advanced technologies have made more apparent to humans in
recent decades is that microbial colonization is very common even in
environments that humans think of as being nearly sterile.
Because it is normal to have bacterial colonization, it is difficult to
know which chronic wounds can be classified as infected and how much
risk of progression exists. Despite the huge number of wounds seen in
clinical practice, there are limited quality data for evaluated symptoms
and signs. A review of chronic wounds in the Journal of the American
Medical Association's "Rational Clinical Examination Series" quantified
the importance of increased pain as an indicator of infection.
The review showed that the most useful finding is an increase in the
level of pain [likelihood ratio (LR) range, 11–20] makes infection much
more likely, but the absence of pain (negative likelihood ratio range,
0.64–0.88) does not rule out infection (summary LR 0.64–0.88).
Disease
Disease can arise if the host's protective immune mechanisms are compromised and the organism inflicts damage on the host. Microorganisms can cause tissue damage by releasing a variety of toxins or destructive enzymes. For example, Clostridium tetani releases a toxin that paralyzes muscles, and staphylococcus
releases toxins that produce shock and sepsis. Not all infectious
agents cause disease in all hosts. For example, less than 5% of
individuals infected with polio develop disease. On the other hand, some infectious agents are highly virulent. The prion causing mad cow disease and Creutzfeldt–Jakob disease invariably kills all animals and people that are infected.
Persistent infections occur because the body is unable to clear
the organism after the initial infection. Persistent infections are
characterized by the continual presence of the infectious organism,
often as latent infection with occasional recurrent relapses of active
infection. There are some viruses that can maintain a persistent
infection by infecting different cells of the body. Some viruses once
acquired never leave the body. A typical example is the herpes virus,
which tends to hide in nerves and become reactivated when specific
circumstances arise.
Persistent infections cause millions of deaths globally each year. Chronic infections by parasites account for a high morbidity and mortality in many underdeveloped countries.
Transmission
A southern house mosquito (Culex quinquefasciatus) is a vector that transmits the pathogens that cause West Nile fever and avian malaria among others.
For
infecting organisms to survive and repeat the infection cycle in other
hosts, they (or their progeny) must leave an existing reservoir and
cause infection elsewhere. Infection transmission can take place via
many potential routes:
- Droplet contact, also known as the respiratory route, and the resultant infection can be termed airborne disease. If an infected person coughs or sneezes on another person the microorganisms, suspended in warm, moist droplets, may enter the body through the nose, mouth or eye surfaces.
- Fecal-oral transmission, wherein foodstuffs or water become contaminated (by people not washing their hands before preparing food, or untreated sewage being released into a drinking water supply) and the people who eat and drink them become infected. Common fecal-oral transmitted pathogens include Vibrio cholerae, Giardia species, rotaviruses, Entameba histolytica, Escherichia coli, and tape worms. Most of these pathogens cause gastroenteritis.
- Sexual transmission, with the resulting disease being called sexually transmitted disease
- Oral transmission, Diseases that are transmitted primarily by oral means may be caught through direct oral contact such as kissing, or by indirect contact such as by sharing a drinking glass or a cigarette.
- Transmission by direct contact, Some diseases that are transmissible by direct contact include athlete's foot, impetigo and warts
- Vehicle transmission, transmission by an inanimate reservoir (food, water, soil).
- Vertical transmission, directly from the mother to an embryo, fetus or baby during pregnancy or childbirth. It can occur when the mother gets an infection as an intercurrent disease in pregnancy.
- Iatrogenic transmission, due to medical procedures such as injection or transplantation of infected material.
- Vector-borne transmission, transmitted by a vector, which is an organism that does not cause disease itself but that transmits infection by conveying pathogens from one host to another.
The relationship between virulence versus transmissibility is complex; if a disease is rapidly fatal, the host may die before the microbe can be passed along to another host.
Diagnosis
Diagnosis
of infectious disease sometimes involves identifying an infectious
agent either directly or indirectly. In practice most minor infectious
diseases such as warts, cutaneous abscesses, respiratory system infections and diarrheal diseases
are diagnosed by their clinical presentation and treated without
knowledge of the specific causative agent. Conclusions about the cause
of the disease are based upon the likelihood that a patient came in
contact with a particular agent, the presence of a microbe in a
community, and other epidemiological considerations. Given sufficient
effort, all known infectious agents can be specifically identified. The
benefits of identification, however, are often greatly outweighed by the
cost, as often there is no specific treatment, the cause is obvious, or
the outcome of an infection is benign.
Diagnosis of infectious disease is nearly always initiated by medical history
and physical examination. More detailed identification techniques
involve the culture of infectious agents isolated from a patient.
Culture allows identification of infectious organisms by examining their
microscopic features, by detecting the presence of substances produced
by pathogens, and by directly identifying an organism by its genotype.
Other techniques (such as X-rays, CAT scans, PET scans or NMR)
are used to produce images of internal abnormalities resulting from the
growth of an infectious agent. The images are useful in detection of,
for example, a bone abscess or a spongiform encephalopathy produced by a prion.
Symptomatic diagnostics
The
diagnosis is aided by the presenting symptoms in any individual with an
infectious disease, yet it usually needs additional diagnostic
techniques to confirm the suspicion. Some signs are specifically
characteristic and indicative of a disease and are called pathognomonic signs; but these are rare. Not all infections are symptomatic.
In children the presence of cyanosis, rapid breathing, poor peripheral perfusion, or a petechial rash increases the risk of a serious infection by greater than 5 fold. Other important indicators include parental concern, clinical instinct, and temperature greater than 40 °C.
Microbial culture
Four nutrient agar plates growing colonies of common Gram negative bacteria.
Microbiological culture is a principal tool used to diagnose infectious disease. In a microbial culture, a growth medium
is provided for a specific agent. A sample taken from potentially
diseased tissue or fluid is then tested for the presence of an
infectious agent able to grow within that medium. Most pathogenic
bacteria are easily grown on nutrient agar, a form of solid medium that supplies carbohydrates and proteins necessary for growth of a bacterium, along with copious amounts of water. A single bacterium will grow into a visible mound on the surface of the plate called a colony,
which may be separated from other colonies or melded together into a
"lawn". The size, color, shape and form of a colony is characteristic of
the bacterial species, its specific genetic makeup (its strain),
and the environment that supports its growth. Other ingredients are
often added to the plate to aid in identification. Plates may contain
substances that permit the growth of some bacteria and not others, or
that change color in response to certain bacteria and not others.
Bacteriological plates such as these are commonly used in the clinical
identification of infectious bacterium. Microbial culture may also be
used in the identification of viruses:
the medium in this case being cells grown in culture that the virus can
infect, and then alter or kill. In the case of viral identification, a
region of dead cells results from viral growth, and is called a
"plaque". Eukaryotic parasites may also be grown in culture as a means of identifying a particular agent.
In the absence of suitable plate culture techniques, some microbes require culture within live animals. Bacteria such as Mycobacterium leprae and Treponema pallidum
can be grown in animals, although serological and microscopic
techniques make the use of live animals unnecessary. Viruses are also
usually identified using alternatives to growth in culture or animals.
Some viruses may be grown in embryonated
eggs. Another useful identification method is Xenodiagnosis, or the use
of a vector to support the growth of an infectious agent. Chagas disease is the most significant example, because it is difficult to directly demonstrate the presence of the causative agent, Trypanosoma cruzi
in a patient, which therefore makes it difficult to definitively make a
diagnosis. In this case, xenodiagnosis involves the use of the vector of the Chagas agent T. cruzi, an uninfected triatomine bug, which takes a blood meal from a person suspected of having been infected. The bug is later inspected for growth of T. cruzi within its gut.
Microscopy
Another principal tool in the diagnosis of infectious disease is microscopy.
Virtually all of the culture techniques discussed above rely, at some
point, on microscopic examination for definitive identification of the
infectious agent. Microscopy may be carried out with simple instruments,
such as the compound light microscope, or with instruments as complex as an electron microscope.
Samples obtained from patients may be viewed directly under the light
microscope, and can often rapidly lead to identification. Microscopy is
often also used in conjunction with biochemical staining techniques, and can be made exquisitely specific when used in combination with antibody based techniques. For example, the use of antibodies made artificially fluorescent (fluorescently labeled antibodies) can be directed to bind to and identify a specific antigens present on a pathogen. A fluorescence microscope
is then used to detect fluorescently labeled antibodies bound to
internalized antigens within clinical samples or cultured cells. This
technique is especially useful in the diagnosis of viral diseases, where
the light microscope is incapable of identifying a virus directly.
Other microscopic procedures may also aid in identifying
infectious agents. Almost all cells readily stain with a number of basic
dyes due to the electrostatic
attraction between negatively charged cellular molecules and the
positive charge on the dye. A cell is normally transparent under a
microscope, and using a stain increases the contrast of a cell with its
background. Staining a cell with a dye such as Giemsa stain or crystal violet
allows a microscopist to describe its size, shape, internal and
external components and its associations with other cells. The response
of bacteria to different staining procedures is used in the taxonomic classification of microbes as well. Two methods, the Gram stain and the acid-fast
stain, are the standard approaches used to classify bacteria and to
diagnosis of disease. The Gram stain identifies the bacterial groups Firmicutes and Actinobacteria,
both of which contain many significant human pathogens. The acid-fast
staining procedure identifies the Actinobacterial genera Mycobacterium and Nocardia.
Biochemical tests
Biochemical tests used in the identification of infectious agents include the detection of metabolic or enzymatic products characteristic of a particular infectious agent. Since bacteria ferment carbohydrates in patterns characteristic of their genus and species, the detection of fermentation products is commonly used in bacterial identification. Acids, alcohols and gases are usually detected in these tests when bacteria are grown in selective liquid or solid media.
The isolation of enzymes
from infected tissue can also provide the basis of a biochemical
diagnosis of an infectious disease. For example, humans can make neither
RNA replicases nor reverse transcriptase, and the presence of these enzymes are characteristic of specific types of viral infections. The ability of the viral protein hemagglutinin to bind red blood cells
together into a detectable matrix may also be characterized as a
biochemical test for viral infection, although strictly speaking
hemagglutinin is not an enzyme and has no metabolic function.
Serological
methods are highly sensitive, specific and often extremely rapid tests
used to identify microorganisms. These tests are based upon the ability
of an antibody to bind specifically to an antigen. The antigen, usually a
protein or carbohydrate made by an infectious agent, is bound by the
antibody. This binding then sets off a chain of events that can be
visibly obvious in various ways, dependent upon the test. For example, "Strep throat" is often diagnosed within minutes, and is based on the appearance of antigens made by the causative agent, S. pyogenes,
that is retrieved from a patient's throat with a cotton swab.
Serological tests, if available, are usually the preferred route of
identification, however the tests are costly to develop and the reagents
used in the test often require refrigeration.
Some serological methods are extremely costly, although when commonly
used, such as with the "strep test", they can be inexpensive.
Complex serological techniques have been developed into what are known as Immunoassays.
Immunoassays can use the basic antibody – antigen binding as the basis
to produce an electro-magnetic or particle radiation signal, which can
be detected by some form of instrumentation. Signal of unknowns can be
compared to that of standards allowing quantitation of the target
antigen. To aid in the diagnosis of infectious diseases, immunoassays
can detect or measure antigens from either infectious agents or proteins
generated by an infected organism in response to a foreign agent. For
example, immunoassay A may detect the presence of a surface protein from
a virus particle. Immunoassay B on the other hand may detect or measure
antibodies produced by an organism's immune system that are made to
neutralize and allow the destruction of the virus.
Instrumentation can be used to read extremely small signals
created by secondary reactions linked to the antibody – antigen binding.
Instrumentation can control sampling, reagent use, reaction times,
signal detection, calculation of results, and data management to yield a
cost effective automated process for diagnosis of infectious disease.
PCR-based diagnostics
Technologies based upon the polymerase chain reaction
(PCR) method will become nearly ubiquitous gold standards of
diagnostics of the near future, for several reasons. First, the catalog
of infectious agents has grown to the point that virtually all of the
significant infectious agents of the human population have been
identified. Second, an infectious agent must grow within the human body
to cause disease; essentially it must amplify its own nucleic acids in
order to cause a disease. This amplification of nucleic acid in infected
tissue offers an opportunity to detect the infectious agent by using
PCR. Third, the essential tools for directing PCR, primers, are derived from the genomes of infectious agents, and with time those genomes will be known, if they are not already.
Thus, the technological ability to detect any infectious agent
rapidly and specifically are currently available. The only remaining
blockades to the use of PCR as a standard tool of diagnosis are in its
cost and application, neither of which is insurmountable. The diagnosis
of a few diseases will not benefit from the development of PCR methods,
such as some of the clostridial diseases (tetanus and botulism).
These diseases are fundamentally biological poisonings by relatively
small numbers of infectious bacteria that produce extremely potent neurotoxins.
A significant proliferation of the infectious agent does not occur,
this limits the ability of PCR to detect the presence of any bacteria.
Metagenomic sequencing
Given
the wide range of bacteria, viruses, and other pathogens that cause
debilitating and life-threatening illness, the ability to quickly
identify the cause of infection is important yet often challenging. For
example, more than half of cases of encephalitis,
a severe illness affecting the brain, remain undiagnosed, despite
extensive testing using state-of-the-art clinical laboratory methods. Metagenomics
is currently being researched for clinical use, and shows promise as a
sensitive and rapid way to diagnose infection using a single
all-encompassing test. This test is similar to current PCR tests;
however, amplification of genetic material is unbiased rather than using
primers for a specific infectious agent. This amplification step is followed by next-generation sequencing and alignment comparisons using large databases of thousands of organismic and viral genomes.
Metagenomic sequencing could prove especially useful for diagnosis when the patient is immunocompromised.
An ever-wider array of infectious agents can cause serious harm to
individuals with immunosuppression, so clinical screening must often be
broader. Additionally, the expression of symptoms is often atypical,
making clinical diagnosis based on presentation more difficult. Thirdly,
diagnostic methods that rely on the detection of antibodies are more
likely to fail. A broad, sensitive test for pathogens that detects the
presence of infectious material rather than antibodies is therefore
highly desirable.
Indication of tests
There is usually an indication
for a specific identification of an infectious agent only when such
identification can aid in the treatment or prevention of the disease, or
to advance knowledge of the course of an illness prior to the
development of effective therapeutic or preventative measures. For
example, in the early 1980s, prior to the appearance of AZT for the treatment of AIDS,
the course of the disease was closely followed by monitoring the
composition of patient blood samples, even though the outcome would not
offer the patient any further treatment options. In part, these studies
on the appearance of HIV in specific communities permitted the advancement of hypotheses
as to the route of transmission of the virus. By understanding how the
disease was transmitted, resources could be targeted to the communities
at greatest risk in campaigns aimed at reducing the number of new
infections. The specific serological diagnostic identification, and later genotypic or molecular identification, of HIV also enabled the development of hypotheses as to the temporal and geographical origins of the virus, as well as a myriad of other hypothesis.
The development of molecular diagnostic tools have enabled physicians
and researchers to monitor the efficacy of treatment with anti-retroviral drugs.
Molecular diagnostics are now commonly used to identify HIV in healthy
people long before the onset of illness and have been used to
demonstrate the existence of people who are genetically resistant to HIV
infection. Thus, while there still is no cure for AIDS, there is great
therapeutic and predictive benefit to identifying the virus and
monitoring the virus levels within the blood of infected individuals,
both for the patient and for the community at large.
Prevention
Washing one's hands, a form of hygiene, is an effective way to prevent the spread of infectious disease.
Techniques like hand washing, wearing gowns, and wearing face masks
can help prevent infections from being passed from one person to
another. Aseptic technique
was introduced in medicine and surgery in the late 19th century and
greatly reduced the incidence of infections caused by surgery. Frequent hand washing remains the most important defense against the spread of unwanted organisms.
There are other forms of prevention such as avoiding the use of illicit
drugs, using a condom, wearing gloves, and having a healthy lifestyle
with a balanced diet and regular exercise. Cooking foods well and
avoiding foods that have been left outside for a long time is also
important.
Antimicrobial substances used to prevent transmission of infections include:
- antiseptics, which are applied to living tissue/skin
- disinfectants, which destroy microorganisms found on non-living objects.
- antibiotics, called prophylactic when given as prevention rather as treatment of infection. However, long term use of antibiotics leads to resistance of bacteria. While humans do not become immune to antibiotics, the bacteria does. Thus, avoiding using antibiotics longer than necessary helps preventing bacteria from forming mutations that aide in antibiotic resistance.
One of the ways to prevent or slow down the transmission of
infectious diseases is to recognize the different characteristics of
various diseases. Some critical disease characteristics that should be evaluated include virulence, distance traveled by victims, and level of contagiousness. The human strains of Ebola
virus, for example, incapacitate their victims extremely quickly and
kill them soon after. As a result, the victims of this disease do not
have the opportunity to travel very far from the initial infection zone. Also, this virus must spread through skin lesions or permeable membranes such as the eye. Thus, the initial stage of Ebola
is not very contagious since its victims experience only internal
hemorrhaging. As a result of the above features, the spread of Ebola is
very rapid and usually stays within a relatively confined geographical
area. In contrast, the Human Immunodeficiency Virus (HIV) kills its victims very slowly by attacking their immune system.
As a result, many of its victims transmit the virus to other
individuals before even realizing that they are carrying the disease.
Also, the relatively low virulence allows its victims to travel long
distances, increasing the likelihood of an epidemic.
Another effective way to decrease the transmission rate of infectious diseases is to recognize the effects of small-world networks.
In epidemics, there are often extensive interactions within hubs or
groups of infected individuals and other interactions within discrete
hubs of susceptible individuals. Despite the low interaction between
discrete hubs, the disease can jump to and spread in a susceptible hub
via a single or few interactions with an infected hub. Thus, infection
rates in small-world networks can be reduced somewhat if interactions
between individuals within infected hubs are eliminated (Figure 1).
However, infection rates can be drastically reduced if the main focus is
on the prevention of transmission jumps between hubs. The use of needle
exchange programs in areas with a high density of drug users with HIV
is an example of the successful implementation of this treatment method.
[6] Another example is the use of ring culling or
vaccination of potentially susceptible livestock in adjacent farms to
prevent the spread of the foot-and-mouth virus in 2001.
A general method to prevent transmission of vector-borne pathogens is pest control.
Immunity
Mary Mallon (a.k.a. Typhoid Mary) was an asymptomatic carrier of typhoid fever. Over the course of her career as a cook, she infected 53 people, three of whom died.
Infection with most pathogens does not result in death of the host
and the offending organism is ultimately cleared after the symptoms of
the disease have waned. This process requires immune mechanisms to kill or inactivate the inoculum of the pathogen. Specific acquired immunity against infectious diseases may be mediated by antibodies and/or T lymphocytes. Immunity mediated by these two factors may be manifested by:
- a direct effect upon a pathogen, such as antibody-initiated complement-dependent bacteriolysis, opsonoization, phagocytosis and killing, as occurs for some bacteria,
- neutralization of viruses so that these organisms cannot enter cells,
- or by T lymphocytes, which will kill a cell parasitized by a microorganism.
The immune system response to a microorganism often causes symptoms such as a high fever and inflammation, and has the potential to be more devastating than direct damage caused by a microbe.
Resistance to infection (immunity) may be acquired following a disease, by asymptomatic carriage of the pathogen, by harboring an organism with a similar structure (crossreacting), or by vaccination.
Knowledge of the protective antigens and specific acquired host immune
factors is more complete for primary pathogens than for opportunistic pathogens.
There is also the phenomenon of herd immunity
which offers a measure of protection to those otherwise vulnerable
people when a large enough proportion of the population has acquired
immunity from certain infections.
Immune resistance to an infectious disease requires a critical
level of either antigen-specific antibodies and/or T cells when the host
encounters the pathogen. Some individuals develop natural serum antibodies to the surface polysaccharides
of some agents although they have had little or no contact with the
agent, these natural antibodies confer specific protection to adults and
are passively transmitted to newborns.
Host genetic factors
The
organism that is the target of an infecting action of a specific
infectious agent is called the host. The host harbouring an agent that
is in a mature or sexually active stage phase is called the definitive
host. The intermediate host comes in contact during the larvae stage. A
host can be anything living and can attain to asexual and sexual
reproduction.
The clearance of the pathogens, either treatment-induced or spontaneous,
it can be influenced by the genetic variants carried by the individual
patients. For instance, for genotype 1 hepatitis C treated with Pegylated interferon-alpha-2a or Pegylated interferon-alpha-2b (brand names Pegasys or PEG-Intron) combined with ribavirin,
it has been shown that genetic polymorphisms near the human IL28B gene,
encoding interferon lambda 3, are associated with significant
differences in the treatment-induced clearance of the virus. This
finding, originally reported in Nature,
showed that genotype 1 hepatitis C patients carrying certain genetic
variant alleles near the IL28B gene are more possibly to achieve
sustained virological response after the treatment than others. Later
report from Nature demonstrated that the same genetic variants are also associated with the natural clearance of the genotype 1 hepatitis C virus.
Treatments
When infection attacks the body, anti-infective
drugs can suppress the infection. Several broad types of anti-infective
drugs exist, depending on the type of organism targeted; they include
antibacterial (antibiotic; including antitubercular), antiviral, antifungal and antiparasitic (including antiprotozoal and antihelminthic)
agents. Depending on the severity and the type of infection, the
antibiotic may be given by mouth or by injection, or may be applied topically. Severe infections of the brain are usually treated with intravenous antibiotics. Sometimes, multiple antibiotics are used in case there is resistance
to one antibiotic. Antibiotics only work for bacteria and do not affect
viruses. Antibiotics work by slowing down the multiplication of
bacteria or killing the bacteria. The most common classes of antibiotics
used in medicine include penicillin, cephalosporins, aminoglycosides, macrolides, quinolones and tetracyclines.
Not all infections require treatment, and for many self-limiting infections the treatment may cause more side-effects than benefits. Antimicrobial stewardship
is the concept that healthcare providers should treat an infection with
an antimicrobial that specifically works well for the target pathogen
for the shortest amount of time and to only treat when there is a known
or highly suspected pathogen that will respond to the medication.
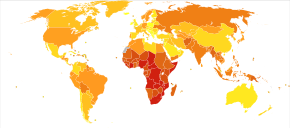
Epidemiology
Deaths due to infectious and parasitic diseases per million persons in 2012
28-81
82-114
115-171
172-212
213-283
284-516
517-1,193
1,194-2,476
2,477-3,954
3,955-6812
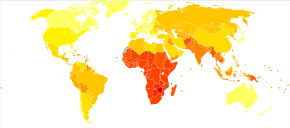
Disability-adjusted life year for infectious and parasitic diseases per 100,000 inhabitants in 2004.
no data
≤250
250–500
500–1000
1000–2000
2000–3000
3000–4000
4000–5000
5000–6250
6250–12,500
12,500–25,000
25,000–50,000
≥50,000
In 2010, about 10 million people died of infectious diseases.
The World Health Organization collects information on global deaths by International Classification of Disease (ICD) code categories. The following table lists the top infectious disease by number of deaths in 2002. 1993 data is included for comparison.
| Rank | Cause of death | Deaths 2002 (in millions) |
Percentage of all deaths |
Deaths 1993 (in millions) |
1993 Rank |
|---|---|---|---|---|---|
| N/A | All infectious diseases | 14.7 | 25.9% | 16.4 | 32.2% |
| 1 | Lower respiratory infections | 3.9 | 6.9% | 4.1 | 1 |
| 2 | HIV/AIDS | 2.8 | 4.9% | 0.7 | 7 |
| 3 | Diarrheal diseases | 1.8 | 3.2% | 3.0 | 2 |
| 4 | Tuberculosis (TB) | 1.6 | 2.7% | 2.7 | 3 |
| 5 | Malaria | 1.3 | 2.2% | 2.0 | 4 |
| 6 | Measles | 0.6 | 1.1% | 1.1 | 5 |
| 7 | Pertussis | 0.29 | 0.5% | 0.36 | 7 |
| 8 | Tetanus | 0.21 | 0.4% | 0.15 | 12 |
| 9 | Meningitis | 0.17 | 0.3% | 0.25 | 8 |
| 10 | Syphilis | 0.16 | 0.3% | 0.19 | 11 |
| 11 | Hepatitis B | 0.10 | 0.2% | 0.93 | 6 |
| 12-17 | Tropical diseases (6) | 0.13 | 0.2% | 0.53 | 9, 10, 16–18 |
| Note: Other causes of death include maternal and perinatal conditions (5.2%), nutritional deficiencies (0.9%), noncommunicable conditions (58.8%), and injuries (9.1%). | |||||
The top three single agent/disease killers are HIV/AIDS, TB and malaria.
While the number of deaths due to nearly every disease have decreased,
deaths due to HIV/AIDS have increased fourfold. Childhood diseases
include pertussis, poliomyelitis, diphtheria, measles and tetanus.
Children also make up a large percentage of lower respiratory and
diarrheal deaths. In 2012, approximately 3.1 million people have died
due to lower respiratory infections, making it the number 4 leading
cause of death in the world.
Historic pandemics
Great Plague of Marseille in 1720 killed 100,000 people in the city and the surrounding provinces
A pandemic (or global epidemic) is a disease that affects people over an extensive geographical area.
- Plague of Justinian, from 541 to 542, killed between 50% and 60% of Europe's population.
- The Black Death of 1347 to 1352 killed 25 million in Europe over 5 years. The plague reduced the old world population from an estimated 450 million to between 350 and 375 million in the 14th century.
- The introduction of smallpox, measles, and typhus to the areas of Central and South America by European explorers during the 15th and 16th centuries caused pandemics among the native inhabitants. Between 1518 and 1568 disease pandemics are said to have caused the population of Mexico to fall from 20 million to 3 million.
- The first European influenza epidemic occurred between 1556 and 1560, with an estimated mortality rate of 20%.
- Smallpox killed an estimated 60 million Europeans during the 18th century (approximately 400,000 per year). Up to 30% of those infected, including 80% of the children under 5 years of age, died from the disease, and one-third of the survivors went blind.
- In the 19th century, tuberculosis killed an estimated one-quarter of the adult population of Europe; by 1918 one in six deaths in France were still caused by TB.
- The Influenza Pandemic of 1918 (or the Spanish Flu) killed 25–50 million people (about 2% of world population of 1.7 billion). Today Influenza kills about 250,000 to 500,000 worldwide each year.
Emerging diseases
In most cases, microorganisms live in harmony with their hosts via mutual or commensal interactions. Diseases can emerge when existing parasites become pathogenic or when new pathogenic parasites enter a new host.
- Coevolution between parasite and host can lead to hosts becoming resistant to the parasites or the parasites may evolve greater virulence, leading to immunopathological disease.
- Human activity is involved with many emerging infectious diseases, such as environmental change enabling a parasite to occupy new niches. When that happens, a pathogen that had been confined to a remote habitat has a wider distribution and possibly a new host organism. Parasites jumping from nonhuman to human hosts are known as zoonoses. Under disease invasion, when a parasite invades a new host species, it may become pathogenic in the new host.
Several human activities have led to the emergence of zoonotic human pathogens, including viruses, bacteria, protozoa, and rickettsia, and spread of vector-borne diseases:
- Encroachment on wildlife habitats. The construction of new villages and housing developments in rural areas force animals to live in dense populations, creating opportunities for microbes to mutate and emerge.
- Changes in agriculture. The introduction of new crops attracts new crop pests and the microbes they carry to farming communities, exposing people to unfamiliar diseases.
- The destruction of rain forests. As countries make use of their rain forests, by building roads through forests and clearing areas for settlement or commercial ventures, people encounter insects and other animals harboring previously unknown microorganisms.
- Uncontrolled urbanization. The rapid growth of cities in many developing countries tends to concentrate large numbers of people into crowded areas with poor sanitation. These conditions foster transmission of contagious diseases.
- Modern transport. Ships and other cargo carriers often harbor unintended "passengers", that can spread diseases to faraway destinations. While with international jet-airplane travel, people infected with a disease can carry it to distant lands, or home to their families, before their first symptoms appear.
History
East German postage stamps depicting four antique microscopes. Advancements in microscopy were essential to the early study of infectious diseases.
Ideas of contagion became more popular in Europe during the Renaissance, particularly through the writing of the Italian physician Girolamo Fracastoro.
Anton van Leeuwenhoek (1632–1723) advanced the science of microscopy by being the first to observe microorganisms, allowing for easy visualization of bacteria.
In the mid-19th century John Snow and William Budd
did important work demonstrating the contagiousness of typhoid and
cholera through contaminated water. Both are credited with decreasing
epidemics of cholera in their towns by implementing measures to prevent
contamination of water.
Louis Pasteur proved beyond doubt that certain diseases are caused by infectious agents, and developed a vaccine for rabies.
Robert Koch, provided the study of infectious diseases with a scientific basis known as Koch's postulates.
Edward Jenner, Jonas Salk and Albert Sabin developed effective vaccines for smallpox and polio, which would later result in the eradication and near-eradication of these diseases, respectively.
Alexander Fleming discovered the world's first antibiotic, Penicillin, which Florey and Chain then developed.
Medical specialists
The medical treatment of infectious diseases falls into the medical field of Infectious Disease and in some cases the study of propagation pertains to the field of Epidemiology. Generally, infections are initially diagnosed by primary care physicians or internal medicine specialists. For example, an "uncomplicated" pneumonia will generally be treated by the internist or the pulmonologist
(lung physician). The work of the infectious diseases specialist
therefore entails working with both patients and general practitioners,
as well as laboratory scientists, immunologists, bacteriologists and other specialists.
An infectious disease team may be alerted when:
- The disease has not been definitively diagnosed after an initial workup
- The patient is immunocompromised (for example, in AIDS or after chemotherapy);
- The infectious agent is of an uncommon nature (e.g. tropical diseases);
- The disease has not responded to first line antibiotics;
- The disease might be dangerous to other patients, and the patient might have to be isolated
Society and culture
A
number of studies have reported associations between pathogen load in
an area and human behavior. Higher pathogen load is associated with
decreased size of ethnic and religious groups in an area. This may be
due high pathogen load favoring avoidance of other groups, which may
reduce pathogen transmission, or a high pathogen load preventing the
creation of large settlements and armies that enforce a common culture.
Higher pathogen load is also associated with more restricted sexual
behavior, which may reduce pathogen transmission. It also associated
with higher preferences for health and attractiveness in mates. Higher fertility rates
and shorter or less parental care per child is another association that
may be a compensation for the higher mortality rate. There is also an
association with polygyny
which may be due to higher pathogen load, making selecting males with a
high genetic resistance increasingly important. Higher pathogen load is
also associated with more collectivism and less individualism, which
may limit contacts with outside groups and infections. There are
alternative explanations for at least some of the associations although
some of these explanations may in turn ultimately be due to pathogen
load. Thus, polygny may also be due to a lower male:female ratio in
these areas but this may ultimately be due to male infants having
increased mortality from infectious diseases. Another example is that
poor socioeconomic factors may ultimately in part be due to high
pathogen load preventing economic development.
Fossil record
Herrerasaurus skull.
Evidence of infection in fossil remains is a subject of interest for paleopathologists,
scientists who study occurrences of injuries and illness in extinct
life forms. Signs of infection have been discovered in the bones of
carnivorous dinosaurs. When present, however, these infections seem to
tend to be confined to only small regions of the body. A skull
attributed to the early carnivorous dinosaur Herrerasaurus ischigualastensis
exhibits pit-like wounds surrounded by swollen and porous bone. The
unusual texture of the bone around the wounds suggests they were
afflicted by a short-lived, non-lethal infection. Scientists who studied
the skull speculated that the bite marks were received in a fight with
another Herrerasaurus. Other carnivorous dinosaurs with documented evidence of infection include Acrocanthosaurus, Allosaurus, Tyrannosaurus and a tyrannosaur from the Kirtland Formation. The infections from both tyrannosaurs were received by being bitten during a fight, like the Herrerasaurus specimen.
Outer space
A 2006 Space Shuttle experiment found that Salmonella typhimurium, a bacterium that can cause food poisoning, became more virulent when cultivated in space. On April 29, 2013, scientists in Rensselaer Polytechnic Institute, funded by NASA, reported that, during spaceflight on the International Space Station, microbes seem to adapt to the space environment in ways "not observed on Earth" and in ways that "can lead to increases in growth and virulence". More recently, in 2017, bacteria were found to be more resistant to antibiotics and to thrive in the near-weightlessness of space. Microorganisms have been observed to survive the vacuum of outer space.