| Angiotensin-converting-enzyme inhibitor | |
|---|---|

Captopril, the first synthetic ACE inhibitor
| |
| Class identifiers | |
| Use | Hypertension |
| ATC code | C09A |
| Biological target | Angiotensin-converting enzyme |
| Clinical data | |
| Drugs.com | Drug Classes |
| Consumer Reports | Best Buy Drugs |
| WebMD | MedicineNet RxList |
| External links | |
| MeSH | D000806 |
| In Wikidata | |
Angiotensin-converting-enzyme inhibitors (ACE inhibitors) are a class of medication used primarily for the treatment of high blood pressure and heart failure. They work by causing relaxation of blood vessels as well as a decrease in blood volume, which leads to lower blood pressure and decreased oxygen demand from the heart.
ACE inhibitors inhibit the activity of angiotensin-converting enzyme, an important component of the renin–angiotensin system liable to convert angiotensin I to angiotensin II, and hydrolyse bradykinin Thereby, ACE inhibitors in turn decrease the formation of angiotensin II, a vasopressin, but increase the level of bradykinin, a peptide vasodilator. This combination, thereby, is synergistic in increasing ACE inhibitors' blood pressure-lowering effect.
Frequently prescribed ACE inhibitors include benazepril, zofenopril, perindopril, trandolapril, captopril, enalapril, lisinopril, and ramipril.
Medical use
ACE
inhibitors were initially approved for the treatment of hypertension
and can be used alone or in combination with other anti-hypertensive
medications. Later, they were found useful for other cardiovascular and
kidney diseases including:
- Acute myocardial infarction (heart attack)
- Heart failure (left ventricular systolic dysfunction)
- Kidney complications of diabetes mellitus (diabetic nephropathy) by means of decreasing the blood pressure and increasing perfusion in glomerular arteriolar.[5]
In treating high blood pressure, ACE inhibitors are often the first drug choice, particularly when diabetes is present,
but age can lead to different choices and it is common to need more
than one drug to obtain the desired improvement. There are fixed-dose combination drugs, such as ACE inhibitor and thiazide combinations. ACE inhibitors have also been used in chronic kidney failure and kidney involvement in systemic sclerosis
(hardening of tissues, as scleroderma renal crisis). In those with
stable coronary artery disease, but no heart failure, benefits are
similar to other usual treatments.
In 2012, there was a meta-analysis published in the BMJ that described the protective role of ACE inhibitors in reducing the risk of pneumonia when compared to ARBs (Angiotensin II Receptor Blockers).
The authors found a decreased risk in patients with previous stroke
(54% risk reduction), with heart failure (37% risk reduction), and of
Asian descent (43% risk reduction vs 54% risk reduction in non-Asian
population). However, no reduced pneumonia related mortality was
observed.
Other
ACE inhibitors may also be used to help decrease excessive water consumption in people with schizophrenia resulting in psychogenic polydipsia. A double-blind, placebo-controlled trial showed that when used for this purpose, enalapril led to decreased consumption (determined by urine output and osmality) in 60% of people; the same effect has been demonstrated in other ACE inhibitors.
Adverse effects
Common side effects include: low blood pressure, cough, hyperkalemia, headache, dizziness, fatigue, nausea, and kidney impairment.
The main adverse effects of ACE inhibition can be understood from
their pharmacological action. The other reported adverse effects are
liver problems and effect on the fetus.
Kidney problems may occur with all ACE inhibitors that directly follows
from their mechanism of action. Patients starting on an ACE inhibitor
usually have a modest reduction in glomerular filtration rate (GFR). However, the decrease may be significant in conditions of pre-existing
decreased renal perfusion, such as renal artery stenosis, heart
failure, polycystic kidney disease, or volume depletion. In these
patients, the maintenance of GFR depends on angiotensin-II-dependent
efferent vasomotor tone. Therefore, renal function
should be closely monitored over the first few days after initiation of
treatment with ACE inhibitor in patients with decreased renal
perfusion. A moderate reduction in renal function, no greater than 30% rise in serum creatinine,
that is stabilized after a week of treatment is deemed acceptable as
part of the therapeutic effect, providing the residual renal function is
sufficient.
Reduced GFR is especially a problem if the patient is concomitantly taking an NSAID and a diuretic. When the three drugs are taken together, the risk of developing renal failure is significantly increased.
High blood potassium
is another possible complication of treatment with an ACE inhibitor due
to its effect on aldosterone. Suppression of angiotensin II leads to a
decrease in aldosterone levels. Since aldosterone is responsible for
increasing the excretion of potassium, ACE inhibitors can cause
retention of potassium. Some people, however, can continue to lose
potassium while on an ACE inhibitor.
Hyperkalemia may decrease the velocity of impulse conduction in the
nerves and muscles, including cardiac tissues. This leads to cardiac
dysfunction and neuromuscular consequences, such as muscle weakness,
paresthesia, nausea, diarrhea, and others. Close monitoring of potassium
levels is required in patients receiving treatment with ACE inhibitors
who are at risk of hyperkalemia.
Another possible adverse effect specific for ACE inhibitors, but not for other RAAS blockers, is an increase in bradykinin level.
A persistent dry cough is a relatively common adverse effect
believed to be associated with the increases in bradykinin levels
produced by ACE inhibitors, although the role of bradykinin in producing
these symptoms has been disputed. Many cases of cough in people on ACE inhibitors may not be from the medication itself, however. People who experience this cough are often switched to angiotensin II receptor antagonists.
Some (0.7%) develop angioedema due to increased bradykinin levels. A genetic predisposition may exist.
A severe rare allergic reaction can affect the bowel wall and secondarily cause abdominal pain.
Blood
Hematologic
effects, such as neutropenia, agranulocytosis and other blood
dyscrasias, have occurred during therapy with ACE inhibitors, especially
in people with additional risk factors.
Pregnancy
In pregnant women, ACE inhibitors taken during all the trimesters have been reported to cause congenital malformations, stillbirths, and neonatal deaths. Commonly reported fetal abnormalities include hypotension, renal dysplasia, anuria/oliguria, oligohydramnios, intrauterine growth retardation, pulmonary hypoplasia, patent ductus arteriosus, and incomplete ossification of the skull. Overall, about half of newborns exposed to ACE inhibitors are adversely affected, leading to birth defects.
ACE inhibitors are ADEC pregnancy category D, and should be avoided in women who are likely to become pregnant. In the U.S., ACE inhibitors must be labeled with a boxed warning
concerning the risk of birth defects when taken during the second and
third trimester. Their use in the first trimester is also associated
with a risk of major congenital malformations, particularly affecting the cardiovascular and central nervous systems.
Overdose
Symptoms
and Treatment: There are few reports of ACE inhibitor overdose in the
literature. The most likely manifestations are hypotension, which may be
severe, hyperkalemia, hyponatremia and renal impairment with metabolic
acidosis. Treatment should be mainly symptomatic and supportive, with
volume expansion using normal saline to correct hypotension and improve
renal function, and gastric lavage followed by activated charcoal and a
cathartic to prevent further absorption of the drug. Captopril,
enalapril, lisinopril and perindopril are known to be removable by
hemodialysis.
Contraindications and precautions
The ACE inhibitors are contraindicated in people with:
- Pregnancy or breastfeeding
- Previous angioedema associated with ACE inhibitor therapy
- Bilateral renal artery stenosis
- Hypersensitivity to ACE inhibitors
ACE inhibitors should be used with caution in people with:
- Impaired renal function
- Aortic valve stenosis or cardiac outflow obstruction
- Hypovolemia or dehydration
- Hemodialysis with high-flux polyacrylonitrile membranes
A combination of ACE inhibitor with other drugs may increase effects of these drugs, but also the risk of adverse effects.
The commonly reported adverse effects of drug combination with ACE are
acute renal failure, hypotension, and hyperkalemia. The drugs
interacting with ACE inhibitor should be prescribed with caution.
Special attention should be given to combinations of ACE inhibitor with
other RAAS blockers, diuretics (especially potassium-sparing diuretics), NSAIDs, anticoagulants, cyclosporine, DPP-4 inhibitors, and potassium supplements.
Potassium supplementation should be used with caution and under medical supervision owing to the hyperkalemic effect of ACE inhibitors.
Concomitant use with cyclooxygenase inhibitors tends to decrease ACE inhibitor's hypotensive effect.
Mechanism of action
ACE
inhibitors reduce the activity of the renin–angiotensin–aldosterone
system (RAAS) as the primary etiologic (causal) event in the development
of hypertension in people with diabetes mellitus, as part of the
insulin-resistance syndrome or as a manifestation of renal disease.
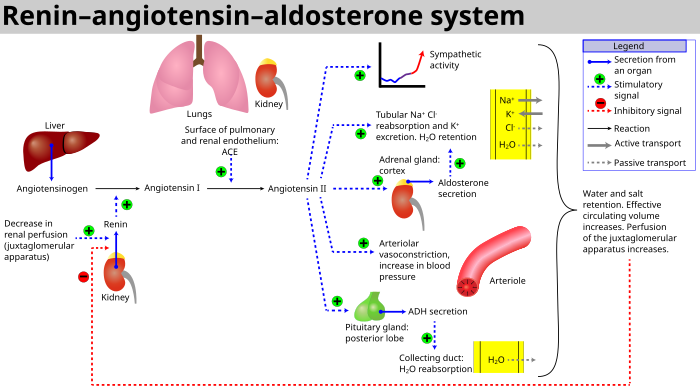
Renin–angiotensin–aldosterone system
Renin–angiotensin–aldosterone system is a major blood pressure
regulating mechanism. Markers of electrolyte and water imbalance in the
body such as hypotension, low distal tubule sodium concentration, decreased blood volume and high sympathetic tone trigger the release of the enzyme renin from the cells of juxtaglomerular apparatus in the kidney. Renin activates a circulating liver derived prohormone angiotensinogen by proteolytic cleavage of all but its first ten amino acid residues known as angiotensin I. ACE (Angiotensin converting enzyme) then removes a further two residues, converting angiotensin I into angiotensin II. ACE is found in the pulmonary circulation and in the endothelium of many blood vessels.
The system increases blood pressure by increasing the amount of salt
and water the body retains, although angiotensin is also very good at
causing the blood vessels to tighten (a potent vasoconstrictor).
Effects
ACE inhibitors block the conversion of Angiotensin I (ATI) to Angiotensin II (ATII).[37] They thereby lower arteriolar resistance and increase venous capacity; decrease cardiac output, cardiac index, stroke work, and volume; lower resistance in blood vessels in the kidneys; and lead to increased natriuresis
(excretion of sodium in the urine).
Renin increases in concentration in the blood as a result of negative
feedback of conversion of ATI to ATII. ATI increases for the same
reason; ATII and aldosterone decrease. Bradykinin increases because of less inactivation by ACE.
Under normal conditions, angiotensin II has these effects:
- Vasoconstriction (narrowing of blood vessels) and vascular smooth muscle hypertrophy (enlargement) induced by ATII may lead to increased blood pressure and hypertension. Further, constriction of the efferent arterioles of the kidney leads to increased perfusion pressure in the glomeruli.
- It contributes to ventricular remodeling and ventricular hypertrophy of the heart through stimulation of the proto-oncogenes c-fos, c-jun, c-myc, transforming growth factor beta (TGF-B), through fibrogenesis and apoptosis (programmed cell death).
- Stimulation by ATII of the adrenal cortex to release aldosterone, a hormone that acts on kidney tubules, causes sodium and chloride ions retention and potassium excretion. Sodium is a "water-holding" ion, so water is also retained, which leads to increased blood volume, hence an increase in blood pressure.
- Stimulation of the posterior pituitary to release vasopressin (antidiuretic hormone, ADH) also acts on the kidneys to increase water retention. If ADH production is excessive in heart failure, Na+ level in the plasma may fall (hyponatremia), and this is a sign of increased risk of death in heart failure patients.
- A decrease renal protein kinase C
During the course of ACE inhibitor use, the production of ATII is decreased, which prevents aldosterone release from the adrenal cortex.
This allows the kidney to excrete sodium ions along with obligate
water, and retain potassium ions. This decreases blood volume, leading
to decreased blood pressure.
Epidemiological and clinical studies have shown ACE inhibitors reduce the progress of diabetic nephropathy independently from their blood pressure-lowering effect. This action of ACE inhibitors is used in the prevention of diabetic renal failure.
ACE inhibitors have been shown to be effective for indications other than hypertension even in patients with normal blood pressure.
The use of a maximum dose of ACE inhibitors in such patients (including
for prevention of diabetic nephropathy, congestive heart failure, and
prophylaxis of cardiovascular events) is justified,
because it improves clinical outcomes independently of the blood
pressure-lowering effect of ACE inhibitors. Such therapy, of course,
requires careful and gradual titration of the dose to prevent the
effects of rapidly decreasing blood pressure (dizziness, fainting,
etc.).
ACE inhibitors have also been shown to cause a central enhancement of parasympathetic nervous system activity in healthy volunteers and patients with heart failure.
This action may reduce the prevalence of malignant cardiac arrhythmias,
and the reduction in sudden death reported in large clinical trials.
ACE Inhibitors also reduce plasma norepinephrine levels, and its resulting vasoconstriction effects, in heart failure patients, thus breaking the vicious circles of sympathetic and renin angiotensin system activation, which sustains the downward spiral in cardiac function in congestive heart failure.
The ACE inhibitor enalapril has also been shown to reduce cardiac cachexia in patients with chronic heart failure. Cachexia is a poor prognostic sign in patients with chronic heart failure.
ACE inhibitors are under early investigation for the treatment of
frailty and muscle wasting (sarcopenia) in elderly patients without
heart failure.
Examples
ACE
inhibitors are easily identifiable by their common suffix, '-pril'. ACE
inhibitors can be divided into three groups based on their molecular
structure of the enzyme binding sites (sulfhydryl, phosphinyl, carboxyl) to the active center of ACE:
Sulfhydryl-containing agents
These agents appear to show antioxidative properties but may be involved in adverse events such as skin eruptions.
Dicarboxylate-containing agents
This is the largest group, including:
- Enalapril (Vasotec/Renitec/Berlipril/Enap/Enalapril Profarma)
- Ramipril (Altace/Prilace/Ramace/Ramiwin/Triatec/Tritace/Ramitac)
- Quinapril (Accupril)
- Perindopril (Coversyl/Aceon/Perindo)
- Lisinopril (Listril/Lopril/Novatec/Prinivil/Zestril, Lisidigal)
- Benazepril (Lotensin)
- Imidapril (Tanatril)
- Trandolapril (Mavik/Odrik/Gopten)
- Cilazapril (Inhibace)
Phosphonate-containing agents
- Fosinopril (Fositen/Monopril) is the only member of this group
Naturally occurring
- A comprehensive resource on anti-hypertensive peptides is available in form of a database. It contains around 1700 unique antihypertensive peptides.
- Arfalasin (HOE 409) is angiotensin antagonist.
Dairy products
- Casokinins and lactokinins, breakdown products of casein and whey, occur naturally after ingestion of milk products, especially cultured milk. Their role in blood pressure control is uncertain.
- The lactotripeptides Val-Pro-Pro and Ile-Pro-Pro produced by the probiotic Lactobacillus helveticus or derived from casein have been shown to have ACE-inhibiting and antihypertensive functions. In one study, L. helveticus PR4 was isolated from Italian cheeses.
Comparative information
All ACE inhibitors have similar antihypertensive efficacy when equivalent doses are administered. The main differences lie with captopril,
the first ACE inhibitor. Captopril has a shorter duration of action and
an increased incidence of adverse effects. It is also the only ACE
inhibitor capable of passing through the blood–brain barrier, although the significance of this characteristic has not been shown to have any positive clinical effects.
In a large clinical study, one of the agents in the ACE inhibitor class, ramipril (Altace), demonstrated an ability to reduce the mortality rates of patients suffering from a myocardial infarction,
and to slow the subsequent development of heart failure. This finding
was made after it was discovered that regular use of ramipril reduced
mortality rates even in test subjects not having suffered from
hypertension.
Some believe ramipril's additional benefits may be shared by some
or all drugs in the ACE-inhibitor class. However, ramipril currently
remains the only ACE inhibitor for which such effects are actually
evidence-based.
A meta-analysis confirmed that ACE inhibitors are effective and
certainly the first-line choice in hypertension treatment. This
meta-analysis was based on 20 trials and a cohort of 158,998 patients,
of whom 91% were hypertensive. ACE inhibitors were used as the active
treatment in seven trials (n=76,615) and angiotensin receptor blocker
(ARB) in 13 trials (n=82,383).
ACE inhibitors were associated with a statistically significant 10%
mortality reduction: (HR 0.90; 95% CI, 0.84–0.97; P=0.004). In contrast,
no significant mortality reduction was observed with ARB treatment (HR
0.99; 95% CI, 0.94–1.04; P=0.683). Analysis of mortality reduction by
different ACE inhibitors showed that perindopril-based regimens are
associated with a statistically significant 13% all-cause mortality
reduction.
Taking into account the broad spectrum of the hypertensive population,
one might expect that an effective treatment with ACE inhibitors, in
particular with perindopril, would result in an important gain of lives saved.
Equivalent doses in hypertension
The
ACE inhibitors have different strengths with different starting
dosages. Dosage should be adjusted according to the clinical response.
| ACE inhibitors dosages for hypertension | |||||
|---|---|---|---|---|---|
| Dosage | Dosage | ||||
| Note: bid = two times a day, tid = three times a day, d = daily Drug dosages from Drug Lookup, Epocrates Online. | |||||
| Name | Equivalent daily dose | Start | Usual | Maximum | |
| Benazepril | 10 mg | 10 mg | 20–40 mg | 80 mg | |
| Captopril | 50 mg (25 mg bid) | 12.5–25 mg bid-tid | 25–50 mg bid-tid | 450 mg/d | |
| Enalapril | 5 mg | 5 mg | 10–40 mg | 40 mg | |
| Fosinopril | 10 mg | 10 mg | 20–40 mg | 80 mg | |
| Lisinopril | 10 mg | 10 mg | 10–40 mg | 80 mg | |
| Moexipril | 7.5 mg | 7.5 mg | 7.5–30 mg | 30 mg | |
| Perindopril | 4 mg | 4 mg | 4–8 mg | 16 mg | |
| Quinapril | 10 mg | 10 mg | 20–80 mg | 80 mg | |
| Ramipril | 2.5 mg | 2.5 mg | 2.5–20 mg | 20 mg | |
| Trandolapril | 2 mg | 1 mg | 2–4 mg | 8 mg | |
Angiotensin II receptor antagonists
ACE inhibitors possess many common characteristics with another class of cardiovascular drugs, angiotensin II receptor antagonists,
which are often used when patients are intolerant of the adverse
effects produced by ACE inhibitors. ACE inhibitors do not completely
prevent the formation of angiotensin II, as blockage is dose-dependent,
so angiotensin II receptor antagonists may be useful because they act to
prevent the action of angiotensin II at the AT1 receptor, leaving AT2 receptor unblocked; the latter may have consequences needing further study.
Use in combination
The
combination therapy of angiotensin II receptor antagonists with ACE
inhibitors may be superior to either agent alone. This combination may
increase levels of bradykinin while blocking the generation of
angiotensin II and its activity at the AT1 receptor. This
'dual blockade' may be more effective than using an ACE inhibitor alone,
because angiotensin II can be generated via non-ACE-dependent pathways.
Preliminary studies suggest this combination of pharmacologic agents
may be advantageous in the treatment of essential hypertension, chronic heart failure, and nephropathy. However, the more recent ONTARGET study showed no benefit of combining the agents and more adverse events.
While statistically significant results have been obtained for its role
in treating hypertension, clinical significance may be lacking. There are warnings about the combination of ACE inhibitors with ARBs.
Patients with heart failure may benefit from the combination in terms of reducing morbidity and ventricular remodeling.
The most compelling evidence for the treatment of nephropathy has been found: This combination therapy partially reversed the proteinuria and also exhibited a renoprotective effect in patients afflicted with diabetic nephropathy, and pediatric IgA nephropathy.
History
The first step in the development of ACE inhibitors was the discovery of ACE in plasma by Leonard T. Skeggs and his colleagues in 1956. Brazilian scientist Sérgio Henrique Ferreira reported a bradykinin-potentiating factor (BPF) present in the venom of Bothrops jararaca, a South American pit viper, in 1965. Ferreira then went to John Vane's
laboratory as a postdoctoral fellow with his already-isolated BPF. The
conversion of the inactive angiotensin I to the potent angiotensin II
was thought to take place in the plasma. However, in 1967, Kevin K. F. Ng and John R. Vane showed plasma ACE is too slow to account for the conversion of angiotensin I to angiotensin II in vivo. Subsequent investigation showed rapid conversion occurs during its passage through the pulmonary circulation.
Bradykinin is rapidly inactivated in the circulating blood, and
it disappears completely in a single pass through the pulmonary
circulation. Angiotensin I also disappears in the pulmonary circulation
because of its conversion to angiotensin II. Furthermore, angiotensin II
passes through the lungs without any loss. The inactivation of
bradykinin and the conversion of angiotensin I to angiotensin II in the
lungs was thought to be caused by the same enzyme.
In 1970, Ng and Vane, using BPF provided by Ferreira, showed the
conversion is inhibited during its passage through the pulmonary
circulation.
BPFs are members of a family of peptides whose potentiating
action is linked to inhibition of bradykinin by ACE. Molecular analysis
of BPF yielded a nonapeptide BPF teprotide (SQ 20,881), which showed the greatest ACE inhibition potency and hypotensive effect in vivo.
Teprotide had limited clinical value as a result of its peptide nature
and lack of activity when given orally. In the early 1970s, knowledge of
the structure-activity relationship required for inhibition of ACE was
growing. David Cushman, Miguel Ondetti
and colleagues used peptide analogues to study the structure of ACE,
using carboxypeptidase A as a model. Their discoveries led to the
development of captopril, the first orally-active ACE inhibitor, in
1975.
Captopril was approved by the United States Food and Drug Administration
in 1981. The first nonsulfhydryl-containing ACE inhibitor, enalapril,
was marketed two years later. At least 12 other ACE inhibitors have
since been marketed.
In 1991, Japanese scientists created the first milk-based ACE
inhibitor, in the form of a fermented milk drink, using specific
cultures to liberate the tripeptide isoleucine-proline-proline (IPP) from the dairy protein. Valine-proline-proline
(VPP) is also liberated in this process—another milk tripeptide with a
very similar chemical structure to IPP. Together, these peptides are now
often referred to as lactotripeptides. In 1996, the first human study confirmed the blood pressure-lowering effect of IPP in fermented milk.
Although twice the amount of VPP is needed to achieve the same
ACE-inhibiting activity as the originally discovered IPP, VPP also is
assumed to add to the total blood pressure lowering effect.
Since the first lactotripeptides discovery, more than 20 human clinical trials have been conducted in many different countries.