From Wikipedia, the free encyclopedia
Boron nitride is a heat and chemically resistant
refractory compound of
boron and
nitrogen with the
chemical formula BN. It exists in various
crystalline forms that are
isoelectronic to a similarly structured
carbon lattice. The
hexagonal form corresponding to
graphite
is the most stable and soft among BN polymorphs, and is therefore used
as a lubricant and an additive to cosmetic products. The cubic (
sphalerite structure) variety analogous to
diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare
wurtzite BN modification is similar to
lonsdaleite and may even be harder than the cubic form.
Because of excellent thermal and chemical stability, boron
nitride ceramics are traditionally used as parts of high-temperature
equipment. Boron nitride has potential use in nanotechnology. Nanotubes
of BN can be produced that have a structure similar to that of
carbon nanotubes, i.e.
graphene (or BN) sheets rolled on themselves, but the properties are very different.
Structure
Boron
nitride exists in multiple forms that differ in the arrangement of the
boron and nitrogen atoms, giving rise to varying bulk properties of the
material.
Amorphous form (a-BN)
The
amorphous form of boron nitride (a-BN) is non-crystalline, lacking any
long-distance regularity in the arrangement of its atoms. It is
analogous to
amorphous carbon.
All other forms of boron nitride are crystalline.
Hexagonal form (h-BN)
The most stable crystalline form is the hexagonal one, also called h-BN, α-BN, g-BN, and
graphitic boron nitride. Hexagonal boron nitride (point group = D
6h; space group = P6
3/mmc) has a layered structure similar to graphite. Within each layer, boron and nitrogen atoms are bound by strong
covalent bonds, whereas the layers are held together by weak
van der Waals forces.
The interlayer "registry" of these sheets differs, however, from the
pattern seen for graphite, because the atoms are eclipsed, with boron
atoms lying over and above nitrogen atoms. This registry reflects the
polarity of the B–N bonds. Still, h-BN and graphite are very close
neighbors and even the BC
6N hybrids have been synthesized where carbon substitutes for some B and N atoms.
Cubic form (c-BN)
Cubic boron nitride has a crystal structure analogous to that of
diamond.
Consistent with diamond being less stable than graphite, the cubic form
is less stable than the hexagonal form, but the conversion rate between
the two is negligible at room temperature, as it is for diamond. The
cubic form has the sphalerite crystal structure, the same as that of
diamond, and is also called β-BN or c-BN.
Wurtzite form (w-BN)
The
wurtzite form of boron nitride (w-BN; point group = C
6v; space group = P6
3mc) has the same structure as
lonsdaleite, a rare hexagonal polymorph of carbon. As in the cubic form, the boron and nitrogen atoms are grouped into
tetrahedra, but in w-BN the angles between neighboring tetrahedra are different. As in the cubic form, the boron and nitrogen atoms are grouped into 6-membered rings; in the cubic form all rings are in the
chair configuration, in w-BN the rings between 'layers' are in
boat configuration.
The Wurtzite form is thought to be very strong, and was estimated by a
simulation as potentially having a strength 18% stronger than that of
diamond, but because only small amounts of the mineral exist in nature,
this has not yet been experimentally verified.
Properties
Physical
Properties of amorphous and crystalline BN, graphite and diamond.
Some properties of h-BN and graphite differ within the basal planes (∥) and perpendicular to them (⟂)
| Material
|
a-BN
|
h-BN
|
c-BN
|
w-BN
|
graphite
|
diamond
|
| Density (g/cm3)
|
2.28
|
~2.1
|
3.45
|
3.49
|
~2.1
|
3.515
|
| Knoop hardness (GPa)
|
10
|
|
45
|
34
|
|
100
|
| Bulk modulus (GPa)
|
100
|
36.5
|
400
|
400
|
34
|
440
|
| Thermal conductivity (W/(m·K))
|
3
|
600 ∥, 30 ⟂
|
740
|
|
200–2000 ∥, 2–800 ⟂
|
600–2000
|
| Thermal expansion (10−6/°C)
|
|
−2.7 ∥, 38 ⟂
|
1.2
|
2.7
|
−1.5 ∥, 25 ⟂
|
0.8
|
| Bandgap (eV)
|
5.05
|
5.2
|
6.4
|
4.5–5.5
|
0
|
5.5
|
| Refractive index
|
1.7
|
1.8
|
2.1
|
2.05
|
|
2.4
|
| Magnetic susceptibility (µemu/g)
|
|
−0.48 ∥, −17.3 ⟂
|
|
|
−0.2...−2.7 ∥, −20...−28 ⟂
|
−1.6
|
The partly
ionic
structure of BN layers in h-BN reduces covalency and electrical
conductivity, whereas the interlayer interaction increases resulting in
higher hardness of h-BN relative to graphite. The reduced
electron-delocalization in hexagonal-BN is also indicated by its absence
of color and a large
band gap. Very different bonding – strong covalent within the
basal planes (planes where boron and nitrogen atoms are covalently bonded) and weak between them – causes high
anisotropy of most properties of h-BN.
For example, the hardness, electrical and thermal conductivity
are much higher within the planes than perpendicular to them. On the
contrary, the properties of c-BN and w-BN are more homogeneous and
isotropic.
Those materials are extremely hard, with the hardness of bulk
c-BN being slightly smaller and w-BN even higher than that of diamond. Polycrystalline c-BN with grain sizes on the order of 10 nm is also reported to have
Vickers hardness comparable or higher than diamond.
Because of much better stability to heat and transition metals, c-BN
surpasses diamond in mechanical applications, such as machining steel. The thermal conductivity of BN is among the highest of all electric insulators.
Boron nitride can be doped p-type with beryllium and n-type with boron, sulfur, silicon or if co-doped with carbon and nitrogen.
Both hexagonal and cubic BN are wide-gap semiconductors with a band-gap
energy corresponding to the UV region. If voltage is applied to h-BN or c-BN, then it emits UV light in the range 215–250 nm and therefore can potentially be used as
light-emitting diodes (LEDs) or lasers.
Little is known on melting behavior of boron nitride. It
sublimates at 2973 °C at normal pressure releasing nitrogen gas and
boron, but melts at elevated pressure.
Thermal stability
Hexagonal
and cubic (and probably w-BN) BN show remarkable chemical and thermal
stabilities. For example, h-BN is stable to decomposition at
temperatures up to 1000 °C in air, 1400 °C in vacuum, and 2800 °C in an
inert atmosphere. The reactivity of h-BN and c-BN is relatively similar,
and the data for c-BN are summarized in the table below.
Reactivity of c-BN with solids
| Solid
|
Ambient
|
Action
|
Threshold T (°C)
|
| Mo
|
10−2 Pa vacuum
|
reaction
|
1360
|
| Ni
|
10−2 Pa vacuum
|
wetting
|
1360
|
| Fe, Ni, Co
|
argon
|
react
|
1400–1500
|
| Al
|
10−2 Pa vacuum
|
wetting and reaction
|
1050
|
| Si
|
10−3 Pa vacuum
|
wetting
|
1500
|
| Cu, Ag, Au, Ga, In, Ge, Sn
|
10−3 Pa vacuum
|
no wetting
|
1100
|
| B
|
|
no wetting
|
2200
|
| Al2O3 + B2O3
|
10−2 Pa vacuum
|
no reaction
|
1360
|
Thermal stability of c-BN can be summarized as follows:
- In air or oxygen: B2O3 protective layer prevents further oxidation to ~1300 °C; no conversion to hexagonal form at 1400 °C.
- In nitrogen: some conversion to h-BN at 1525 °C after 12 h.
- In vacuum (10−5 Pa): conversion to h-BN at 1550–1600 °C.
Chemical stability
Boron nitride is insoluble in the usual acids, but is soluble in alkaline molten salts and nitrides, such as
LiOH,
KOH,
NaOH-
Na2CO3,
NaNO3,
Li3N,
Mg3N2,
Sr3N2,
Ba3N2 or
Li3BN2, which are therefore used to etch BN.
Thermal conductivity
The
theoretical thermal conductivity of hexagonal Boron nitride nanoribbons
(BNNRs) can approach 1700–2000 W/(m·K), which has the same order of
magnitude as the experimental measured value for
graphene, and can be comparable to the theoretical calculations for graphene nanoribbons.
Moreover, the thermal transport in the BNNRs is anisotropic. The
thermal conductivity of zigzag-edged BNNRs is about 20% larger than that
of armchair-edged nanoribbons at room temperature.
Natural occurrence
In 2009, a naturally occurring boron nitride mineral in the cubic form (c-BN) was reported in
Tibet, with a proposed name of
qingsongite. The substance was found in dispersed
micron-sized
inclusions in chromium-rich rocks. In 2013, the International
Mineralogical Association affirmed the mineral and the name.
Synthesis
Preparation and reactivity of hexagonal BN
Boron nitride is produced synthetically. Hexagonal boron nitride is obtained by the reacting boron trioxide (B
2O
3) or boric acid (H
3BO
3) with
ammonia (NH
3) or
urea (CO(NH
2)
2) in a nitrogen atmosphere:
- B2O3 + 2 NH3 → 2 BN + 3 H2O (T = 900 °C)
- B(OH)3 + NH3 → BN + 3 H2O (T = 900 °C)
- B2O3 + CO(NH2)2 → 2 BN + CO2 + 2 H2O (T > 1000 °C)
- B2O3 + 3 CaB6 + 10 N2 → 20 BN + 3 CaO (T > 1500 °C)
The resulting disordered (
amorphous) boron nitride contains 92–95% BN and 5–8% B
2O
3. The remaining B
2O
3 can be evaporated in a second step at temperatures
> 1500 °C
in order to achieve BN concentration >98%. Such annealing also
crystallizes BN, the size of the crystallites increasing with the
annealing temperature.
h-BN parts can be fabricated inexpensively by hot-pressing with
subsequent machining. The parts are made from boron nitride powders
adding boron oxide for better compressibility. Thin films of boron
nitride can be obtained by
chemical vapor deposition from
boron trichloride and nitrogen precursors. Combustion of boron powder in nitrogen
plasma at 5500 °C yields
ultrafine boron nitride used for lubricants and
toners.
- Li3N + BN → Li3BN2
Intercalation of hexagonal BN
Structure of hexagonal boron nitride intercalated with potassium (B4N4K)
Similar to graphite, various molecules, such as NH3 or alkali metals,
can be intercalated into hexagonal boron nitride, that is inserted
between its layers. Both experiment and theory suggest the intercalation
is much more difficult for BN than for graphite.
Preparation of cubic BN
Synthesis
of c-BN uses same methods as that of diamond: Cubic boron nitride is
produced by treating hexagonal boron nitride at high pressure and
temperature, much as
synthetic diamond
is produced from graphite. Direct conversion of hexagonal boron nitride
to the cubic form has been observed at pressures between 5 and 18 GPa
and temperatures between 1730 and 3230 °C, that is similar parameters as
for direct graphite-diamond conversion.
The addition of a small amount of boron oxide can lower the required
pressure to 4–7 GPa and temperature to 1500 °C. As in diamond synthesis,
to further reduce the conversion pressures and temperatures, a catalyst
is added, such as lithium, potassium, or magnesium, their nitrides,
their fluoronitrides, water with ammonium compounds, or hydrazine.
Other industrial synthesis methods, again borrowed from diamond growth,
use crystal growth in a temperature gradient, or explosive
shock wave. The shock wave method is used to produce material called
heterodiamond, a superhard compound of boron, carbon, and nitrogen.
Preparation of wurtzite BN
Wurtzite BN can be obtained via static high-pressure or dynamic shock methods.
The limits of its stability are not well defined. Both c-BN and w-BN
are formed by compressing h-BN, but formation of w-BN occurs at much
lower temperatures close to 1700 °C.
Production statistics
Whereas
the production and consumption figures for the raw materials used for
BN synthesis, namely boric acid and boron trioxide, are well known,
the corresponding numbers for the boron nitride are not listed in
statistical reports. An estimate for the 1999 world production is 300 to
350 metric tons. The major producers and consumers of BN are located in
the United States, Japan, China and Germany. In 2000, prices varied
from about $75/kg to $120/kg for standard industrial-quality h-BN and
were about up to $200–$400/kg for high purity BN grades.
Applications
Hexagonal BN
Hexagonal BN (h-BN) is the most widely used polymorph. It is a good
lubricant at both low and high temperatures (up to 900 °C, even in an
oxidizing atmosphere). h-BN lubricant is particularly useful when the
electrical conductivity or chemical reactivity of graphite (alternative
lubricant) would be problematic. Another advantage of h-BN over graphite
is that its lubricity does not require water or gas molecules trapped
between the layers. Therefore, h-BN lubricants can be used even in
vacuum, e.g. in space applications. The lubricating properties of
fine-grained h-BN are used in
cosmetics,
paints,
dental cements, and
pencil leads.
Hexagonal BN was first used in cosmetics around 1940 in
Japan.
However, because of its high price, h-BN was soon abandoned for this
application. Its use was revitalized in the late 1990s with the
optimization h-BN production processes, and currently h-BN is used by
nearly all leading producers of cosmetic products for
foundations,
make-up,
eye shadows, blushers,
kohl pencils,
lipsticks and other skincare products.
Because of its excellent thermal and chemical stability, boron
nitride ceramics are traditionally used as parts of high-temperature
equipment. h-BN can be included in ceramics, alloys, resins, plastics,
rubbers, and other materials, giving them self-lubricating properties.
Such materials are suitable for construction of e.g.
bearings and in steelmaking.
Plastics filled with BN have less thermal expansion as well as higher
thermal conductivity and electrical resistivity. Due to its excellent
dielectric and thermal properties, BN is used in electronics e.g. as a
substrate for semiconductors, microwave-transparent windows and as a
structural material for seals. It can also be used as dielectric in resistive random access memories.
Hexagonal BN is used in
xerographic process and
laser printers as a charge leakage barrier layer of the photo drum. In the automotive industry, h-BN mixed with a binder (boron oxide) is used for sealing
oxygen sensors,
which provide feedback for adjusting fuel flow. The binder utilizes the
unique temperature stability and insulating properties of h-BN.
Parts can be made by
hot pressing from four commercial grades of h-BN. Grade HBN contains a
boron oxide binder;
it is usable up to 550–850 °C in oxidizing atmosphere and up to 1600 °C
in vacuum, but due to the boron oxide content is sensitive to water.
Grade HBR uses a
calcium borate binder and is usable at 1600 °C. Grades HBC and HBT contain no binder and can be used up to 3000 °C.
Boron nitride nanosheets (h-BN) can be deposited by catalytic decomposition of
borazine at a temperature ~1100 °C in a
chemical vapor deposition setup, over areas up to about 10 cm
2.
Owing to their hexagonal atomic structure, small lattice mismatch with
graphene (~2%), and high uniformity they are used as substrates for
graphene-based devices. BN nanosheets are also excellent
proton conductors. Their high proton transport rate, combined with the high electrical resistance, may lead to applications in
fuel cells and
water electrolysis.
h-BN has been used since the mid-2000s as a bullet and bore
lubricant in precision target rifle applications as an alternative to
molybdenum disulfide
coating, commonly referred to as "moly". It is claimed to increase
effective barrel life, increase intervals between bore cleaning, and
decrease the deviation in point of impact between clean bore first shots
and subsequent shots.
Cubic boron nitride
Cubic boron nitride (CBN or c-BN) is widely used as an
abrasive. Its usefulness arises from its insolubility in
iron,
nickel, and related
alloys
at high temperatures, whereas diamond is soluble in these metals.
Polycrystalline c-BN (PCBN) abrasives are therefore used for machining
steel, whereas diamond abrasives are preferred for aluminum alloys,
ceramics, and stone. When in contact with oxygen at high temperatures,
BN forms a passivation layer of boron oxide. Boron nitride binds well
with metals, due to formation of interlayers of metal borides or
nitrides. Materials with cubic boron nitride crystals are often used in
the
tool bits of
cutting tools.
For grinding applications, softer binders, e.g. resin, porous ceramics,
and soft metals, are used. Ceramic binders can be used as well.
Commercial products are known under names "
Borazon" (by Diamond Innovations), and "Elbor" or "Cubonite" (by Russian vendors).
Contrary to diamond, large c-BN pellets can be produced in a
simple process (called sintering) of annealing c-BN powders in nitrogen
flow at temperatures slightly below the BN decomposition temperature.
This ability of c-BN and h-BN powders to fuse allows cheap production of
large BN parts.
Similar to diamond, the combination in c-BN of highest thermal conductivity and electrical resistivity is ideal for
heat spreaders.
As cubic boron nitride consists of light atoms and is very robust
chemically and mechanically, it is one of the popular materials for
X-ray membranes: low mass results in small X-ray absorption, and good
mechanical properties allow usage of thin membranes, thus further
reducing the absorption.
Amorphous boron nitride
Layers of amorphous boron nitride (a-BN) are used in some
semiconductor devices, e.g.
MOSFETs. They can be prepared by chemical decomposition of
trichloroborazine with
caesium,
or by thermal chemical vapor deposition methods. Thermal CVD can be
also used for deposition of h-BN layers, or at high temperatures, c-BN.
Other forms of boron nitride
Atomically thin boron nitride
Hexagonal
boron nitride can be exfoliated to mono or few atomic layer sheets. Due
to its analogous structure to that of graphene but white appearance,
atomically thin boron nitride is sometimes call “white graphene”.
Mechanical properties. Atomically thin boron nitride is
one of the strongest electrically insulating materials. Monolayer boron
nitride has an average Young's modulus of 0.865TPa and fracture strength
of 70.5GPa, and in contrast to graphene, whose strength decreases
dramatically with increased thickness, few-layer boron nitride sheets
have a strength similar to that of monolayer boron nitride.
Thermal conductivity. Atomically thin boron nitride has
one of the highest thermal conductivity coefficients among
semiconductors and electrical insulators, and its thermal conductivity
increases with reduced thickness due to less intra-layer coupling.
Thermal stability. The air stability of graphene shows a
clear thickness dependence: monolayer graphene is reactive to oxygen at
250 °C, strongly doped at 300 °C, and etched at 450 °C; in contrast,
bulk graphite is not oxidized until 800 °C.
Atomically thin boron nitride has much better oxidation resistance than
graphene. Monolayer boron nitride is not oxidized till 700 °C and can
sustain up to 850 °C in air; bilayer and trilayer boron nitride
nanosheets have slightly higher oxidation starting temperatures.
The excellent thermal stability, high impermeability to gas and liquid,
and electrical insulation make atomically thin boron nitride potential
coating materials for preventing surface oxidation and corrosion of
metals and other two-dimensional (2D) materials, such as black phosphorus.
Better surface adsorption. Atomically thin boron nitride has been found to have better surface adsorption capabilities than bulk hexagonal boron nitride.
According to theoretical and experimental studies, atomically thin
boron nitride as an adsorbent experiences conformational changes upon
surface adsorption of molecules, increasing adsorption energy and
efficiency. The synergic effect of the atomic thickness, high
flexibility, stronger surface adsorption capability, electrical
insulation, impermeability, high thermal and chemical stability of BN
nanosheets can increase the Raman sensitivity by up to two orders, and
in the meantime attain long-term stability and extraordinary reusability
not achievable by other materials.
Dielectric properties. Atomically thin hexagonal boron nitride is an excellent dielectric substrate for graphene, molybdenum disulphide (MoS2),
and many other 2D material-based electronic and photonic devices. As
shown by electric force microscopy (EFM) studies, the electric field
screening in atomically thin boron nitride shows a weak dependence on
thickness, which is in line with the smooth decay of electric field
inside few-layer boron nitride revealed by the first-principles
calculations.
Raman characteristics. Raman spectroscopy has been a useful
tool to study a variety of 2D materials, and the Raman signature of
high-quality atomically thin boron nitride was first reported by
Gorbachev et al. and Li et al.
However, the two reported Raman results of monolayer boron nitride did
not agree with each other. Cai et al., therefore, conducted systematic
experimental and theoretical studies to reveal the intrinsic Raman
spectrum of atomically thin boron nitride.
It reveals that atomically thin boron nitride without interaction with a
substrate has a G band frequency similar to that of bulk hexagonal
boron nitride, but strain induced by the substrate can cause Raman
shifts. Nevertheless, the Raman intensity of G band of atomically thin
boron nitride can be used to estimate layer thickness and sample
quality.
Top: absorption of cyclohexane by BN aerogel. Cyclohexane is stained with Sudan II red dye and is floating on water. Bottom: reuse of the aerogel after burning in air.
Boron nitride nanomesh
Boron nitride nanomesh is a nanostructured two-dimensional material. It consists of a single BN layer, which forms by
self-assembly a highly regular mesh after high-temperature exposure of a clean
rhodium or
ruthenium surface to
borazine under
ultra-high vacuum.
The nanomesh looks like an assembly of hexagonal pores. The distance
between two pore centers is 3.2 nm and the pore diameter is ~2 nm. Other
terms for this material are boronitrene or white graphene.
The boron nitride nanomesh is not only stable to decomposition under vacuum, air and some liquids, but also up to temperatures of 800 °C. In addition, it shows the extraordinary ability to trap
molecules and metallic
clusters
which have similar sizes to the nanomesh pores, forming a well-ordered
array. These characteristics promise interesting applications of the
nanomesh in areas like catalysis,
surface functionalisation,
spintronics,
quantum computing and data storage media like
hard drives.
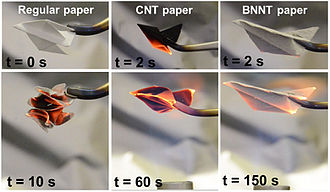
BN nanotubes are flame resistant, as shown in this comparative test of airplanes made of cellullose, carbon buckypaper and BN nanotube buckypaper.
Boron nitride nanotubes
Boron nitride tubules were first made in 1989 by Shore and Dolan This
work was patented in 1989 and published in 1989 thesis (Dolan) and then
1993 Science. The 1989 work was also the first preparation of
amorphous BN by B-trichloroborazine and cesium metal.
Preparation of Amorphous Boron Nitride and Its Conversion to a Turbostratic, Tubular Form Ewan J. M. Hamilton1,
Shawn E. Dolan1,
Charles M. Mann1,
Hendrik O. Colijn2,
Clare A. McDonald2,
Sheldon G. Shore, Science 30 Apr 1993:
Vol. 260, Issue 5108, pp. 659–661
DOI: 10.1126/science.260.5108.659
Boron nitride nanotubes were predicted in 1994 and experimentally discovered in 1995. They can be imagined as a rolled up sheet of h-boron nitride. Structurally, it is a close analog of the
carbon nanotube,
namely a long cylinder with diameter of several to hundred nanometers
and length of many micrometers, except carbon atoms are alternately
substituted by nitrogen and boron atoms. However, the properties of BN
nanotubes are very different: whereas carbon nanotubes can be metallic
or semiconducting depending on the rolling direction and radius, a BN
nanotube is an electrical insulator with a bandgap of ~5.5 eV, basically
independent of tube chirality and morphology. In addition, a layered BN structure is much more thermally and chemically stable than a graphitic carbon structure.
Boron nitride aerogel
Boron nitride aerogel is an
aerogel made of highly porous BN. It typically consists of a mixture of deformed BN nanotubes and
nanosheets. It can have a density as low as 0.6 mg/cm
3 and a specific surface area as high as 1050 m
2/g, and therefore has potential applications as an
absorbent, catalyst support and gas storage medium. BN aerogels are highly
hydrophobic
and can absorb up to 160 times their weight in oil. They are resistant
to oxidation in air at temperatures up to 1200 °C, and hence can be
reused after the absorbed oil is burned out by flame. BN aerogels can be
prepared by template-assisted
chemical vapor deposition using
borazine as the feed gas.
Composites containing BN
Health issues
Boron nitride (along with Si
3N
4, NbN, and BNC) is reported to show weak
fibrogenic activity, and to cause
pneumoconiosis when inhaled in particulate form. The maximum concentration recommended for nitrides of nonmetals is 10 mg/m
3 for BN and 4 for AlN or ZrN.