Anatomy of a multipolar neuron
A neural circuit is a population of neurons interconnected by synapses to carry out a specific function when activated. Neural circuits interconnect to one another to form large scale brain networks. Biological neural networks have inspired the design of artificial neural networks.
Early study
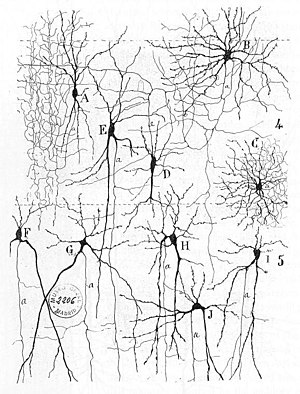
From "Texture of the Nervous System of Man and the Vertebrates" by Santiago Ramón y Cajal. The figure illustrates the diversity of neuronal morphologies in the auditory cortex.
Early treatments of neural networks can be found in Herbert Spencer's Principles of Psychology, 3rd edition (1872), Theodor Meynert's Psychiatry (1884), William James' Principles of Psychology (1890), and Sigmund Freud's Project for a Scientific Psychology (composed 1895). The first rule of neuronal learning was described by Hebb in 1949, in the Hebbian theory.
Thus, Hebbian pairing of pre-synaptic and post-synaptic activity can
substantially alter the dynamic characteristics of the synaptic
connection and therefore either facilitate or inhibit signal transmission. In 1959, the neuroscientists, Warren Sturgis McCulloch and Walter Pitts published the first works on the processing of neural networks. They showed theoretically that networks of artificial neurons could implement logical, arithmetic, and symbolic functions. Simplified models of biological neurons were set up, now usually called perceptrons or artificial neurons. These simple models accounted for neural summation (i.e., potentials at the post-synaptic membrane will summate in the cell body). Later models also provided for excitatory and inhibitory synaptic transmission.
Connections between neurons
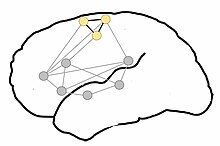
Proposed
organization of motor-semantic neural circuits for action language
comprehension. Gray dots represent areas of language comprehension,
creating a network for comprehending all language. The semantic circuit
of the motor system, particularly the motor representation of the legs
(yellow dots), is incorporated when leg-related words are comprehended.
Adapted from Shebani et al. (2013)
The connections between neurons in the brain are much more complex than those of the artificial neurons used in the connectionist neural computing models of artificial neural networks. The basic kinds of connections between neurons are synapses, chemical and electrical synapses.
The establishment of synapses enables the connection of neurons into millions of overlapping, and interlinking neural circuits. Neurexins are central to this process.
One principle by which neurons work is neural summation – potentials at the postsynaptic membrane will sum up in the cell body. If the depolarization
of the neuron at the axon goes above threshold an action potential will
occur that travels down the axon to the terminal endings to transmit a
signal to other neurons. Excitatory and inhibitory synaptic transmission
is realized mostly by inhibitory postsynaptic potentials (IPSPs) and excitatory postsynaptic potentials (EPSPs).
On the electrophysiological level, there are various phenomena which alter the response characteristics of individual synapses (called synaptic plasticity) and individual neurons (intrinsic plasticity).
These are often divided into short-term plasticity and long-term
plasticity. Long-term synaptic plasticity is often contended to be the
most likely memory substrate. Usually the term "neuroplasticity" refers to changes in the brain that are caused by activity or experience.
Connections display temporal and spatial characteristics.
Temporal characteristics refer to the continuously modified
activity-dependent efficacy of synaptic transmission, called spike-timing-dependent plasticity.
It has been observed in several studies that the synaptic efficacy of
this transmission can undergo short-term increase (called facilitation) or decrease (depression) according to the activity of the presynaptic neuron. The induction of long-term changes in synaptic efficacy, by long-term potentiation (LTP) or depression (LTD), depends strongly on the relative timing of the onset of the excitatory postsynaptic potential
and the postsynaptic action potential. LTP is induced by a series of
action potentials which cause a variety of biochemical responses.
Eventually, the reactions cause the expression of new receptors on the
cellular membranes of the postsynaptic neurons or increase the efficacy
of the existing receptors through phosphorylation.
Backpropagating action potentials cannot occur because after an action potential travels down a given segment of the axon, the m gates on voltage-gated sodium channels close, thus blocking any transient opening of the h gate from causing a change in the intracellular sodium ion (Na+) concentration, and preventing the generation of an action potential back towards the cell body. In some cells, however, neural backpropagation does occur through the dendritic branching and may have important effects on synaptic plasticity and computation.
A neuron in the brain requires a single signal to a neuromuscular
junction to stimulate contraction of the postsynaptic muscle cell. In
the spinal cord, however, at least 75 afferent
neurons are required to produce firing. This picture is further
complicated by variation in time constant between neurons, as some cells
can experience their EPSPs over a wider period of time than others.
While in synapses in the developing brain
synaptic depression has been particularly widely observed it has been
speculated that it changes to facilitation in adult brains.
Circuitry
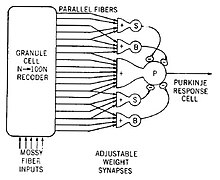
Model of a neural circuit in the cerebellum
An example of a neural circuit is the trisynaptic circuit in the hippocampus. Another is the Papez circuit linking the hypothalamus to the limbic lobe. There are several neural circuits in the cortico-basal ganglia-thalamo-cortical loop. These circuits carry information between the cortex, basal ganglia, thalamus, and back to the cortex. The largest structure within the basal ganglia, the striatum, is seen as having its own internal microcircuitry.
Neural circuits in the spinal cord called central pattern generators are responsible for controlling motor instructions involved in rhythmic behaviours. Rhythmic behaviours include walking, urination, and ejaculation. The central pattern generators are made up of different groups of spinal interneurons.
There are four principal types of neural circuits that are
responsible for a broad scope of neural functions. These circuits are a diverging circuit, a converging circuit, a reverberating circuit, and a parallel after-discharge circuit.
In a diverging circuit, one neuron synapses with a number of
postsynaptic cells. Each of these
may synapse with many more making it possible for one neuron to
stimulate up to thousands of cells. This is exemplified in the way that
thousands of muscle fibers can be stimulated from the initial input from
a single motor neuron.
In a converging circuit, inputs from many sources are converged
into one output, affecting just one neuron or a neuron pool. This type
of circuit is exemplified in the respiratory center of the brainstem, which responds to a number of inputs from different sources by giving out an appropriate breathing pattern.
A reverberating circuit produces a repetitive output. In a
signalling procedure from one neuron to another in a linear sequence,
one of the neurons may send a signal back to initiating neuron.
Each time that the first neuron fires, the other neuron further down the
sequence fires again sending it back to the source. This restimulates
the first neuron and also allows the path of transmission to continue to
its output. A resulting repetitive pattern is the outcome that only
stops if one or more of the synapses fail, or if an inhibitory feed from
another source causes it to stop. This type of reverberating circuit is
found in the respiratory center that sends signals to the respiratory muscles,
causing inhalation. When the circuit is interrupted by an inhibitory
signal the muscles relax causing exhalation. This type of circuit may
play a part in epileptic seizures.
In a parallel after-discharge circuit, a neuron inputs to several
chains of neurons. Each chain is made up of a different number of
neurons but their signals converge onto one output neuron. Each synapse
in the circuit acts to delay the signal by about 0.5 msec so that the
more synapses there are will produce a longer delay to the output
neuron. After the input has stopped, the output will go on firing for
some time. This type of circuit does not have a feedback loop as does
the reverberating circuit. Continued firing after the stimulus has
stopped is called after-discharge. This circuit type is found in the reflex arcs of certain reflexes.
Study methods
Different neuroimaging
techniques have been developed to investigate the activity of neural
circuits and networks. The use of "brain scanners" or functional
neuroimaging to investigate the structure or function of the brain is
common, either as simply a way of better assessing brain injury with
high resolution pictures, or by examining the relative activations of
different brain areas. Such technologies may include functional magnetic resonance imaging (fMRI), brain positron emission tomography (brain PET), and computed axial tomography (CAT) scans. Functional neuroimaging
uses specific brain imaging technologies to take scans from the brain,
usually when a person is doing a particular task, in an attempt to
understand how the activation of particular brain areas is related to
the task. In functional neuroimaging, especially fMRI, which measures hemodynamic activity (using BOLD-contrast imaging) which is closely linked to neural activity, PET, and electroencephalography (EEG) is used.
Connectionist
models serve as a test platform for different hypotheses of
representation, information processing, and signal transmission.
Lesioning studies in such models, e.g. artificial neural networks,
where parts of the nodes are deliberately destroyed to see how the
network performs, can also yield important insights in the working of
several cell assemblies. Similarly, simulations of dysfunctional
neurotransmitters in neurological conditions (e.g., dopamine in the
basal ganglia of Parkinson's
patients) can yield insights into the underlying mechanisms for
patterns of cognitive deficits observed in the particular patient group.
Predictions from these models can be tested in patients or via
pharmacological manipulations, and these studies can in turn be used to
inform the models, making the process iterative.
Clinical significance
Sometimes neural circuitries can become pathological and cause problems such as in Parkinson's disease when the basal ganglia are involved. Problems in the Papez circuit can also give rise to a number of neurodegenerative disorders including Parkinson's.