From Wikipedia, the free encyclopedia
| Battery | |
|---|---|

|
|
| Type | Power source |
| Working principle | Electrochemical reactions, Electromotive force |
| First production | 1800s |
| Electronic symbol | |
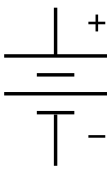 The symbol for a battery in a circuit diagram. It originated as a schematic drawing of the earliest type of battery, a voltaic pile. |
|
An electric battery is a device consisting of one or more electrochemical cells that convert stored chemical energy into electrical energy. Each cell contains a positive terminal, or cathode, and a negative terminal, or anode. Electrolytes allow ions to move between the electrodes and terminals, which allows current to flow out of the battery to perform work.[1]
Primary (single-use or "disposable") batteries are used once and discarded; the electrode materials are irreversibly changed during discharge. Common examples are the alkaline battery used for flashlights and a multitude of portable devices. Secondary (rechargeable batteries) can be discharged and recharged multiple times; the original composition of the electrodes can be restored by reverse current. Examples include the lead-acid batteries used in vehicles and lithium ion batteries used for portable electronics.
Batteries come in many shapes and sizes, from miniature cells used to power hearing aids and wristwatches to battery banks the size of rooms that provide standby power for telephone exchanges and computer data centers.
According to a 2005 estimate, the worldwide battery industry generates US$48 billion in sales each year,[2] with 6% annual growth.
Batteries have much lower specific energy (energy per unit mass) than common fuels such as gasoline. This is somewhat offset by the higher efficiency of electric motors in producing mechanical work, compared to combustion engines.
History
The usage of "battery" to describe a group of electrical devices dates to Benjamin Franklin, who in 1748 described multiple Leyden jars by analogy to a battery of cannon[3] (Benjamin Franklin borrowed the term "battery" from the military, which refers to weapons functioning together[4]).Alessandro Volta described the first electrochemical battery, the voltaic pile in 1800.[5] This was a stack of copper and zinc plates, separated by brine soaked paper disks, that could produce a steady current for a considerable length of time. Volta did not appreciate that the voltage was due to chemical reactions. He thought that his cells were an inexhaustible source of energy,[6] and that the associated corrosion effects at the electrodes were a mere nuisance, rather than an unavoidable consequence of their operation, as Michael Faraday showed in 1834.[7]Although early batteries were of great value for experimental purposes, in practice their voltages fluctuated and they could not provide a large current for a sustained period. The Daniell cell, invented in 1836 by British chemist John Frederic Daniell, was the first practical source of electricity, becoming an industry standard and seeing widespread adoption as a power source for electrical telegraph networks.[8] It consisted of a copper pot filled with a copper sulfate solution, in which was immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode.[9]
These wet cells used liquid electrolytes, which were prone to leakage and spillage if not handled correctly. Many used glass jars to hold their components, which made them fragile. These characteristics made wet cells unsuitable for portable appliances. Near the end of the nineteenth century, the invention of dry cell batteries, which replaced the liquid electrolyte with a paste, made portable electrical devices practical.[10]
Principle of operation
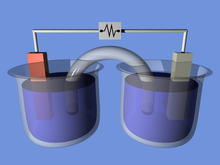
A voltaic cell for demonstration purposes. In this example the two half-cells are linked by a salt bridge separator that permits the transfer of ions.
Batteries convert chemical energy directly to electrical energy. A battery consists of some number of voltaic cells. Each cell consists of two half-cells connected in series by a conductive electrolyte containing anions and cations. One half-cell includes electrolyte and the negative electrode, the electrode to which anions (negatively charged ions) migrate; the other half-cell includes electrolyte and the positive electrode to which cations (positively charged ions) migrate. Redox reactions power the battery. Cations are reduced (electrons are added) at the cathode during charging, while anions are oxidized (electrons are removed) at the anode during discharge.[11] The electrodes do not touch each other, but are electrically connected by the electrolyte. Some cells use different electrolytes for each half-cell. A separator allows ions to flow between half-cells, but prevents mixing of the electrolytes.
Each half-cell has an electromotive force (or emf), determined by its ability to drive electric current from the interior to the exterior of the cell. The net emf of the cell is the difference between the emfs of its half-cells.[12] Thus, if the electrodes have emfs
The electrical driving force or
An ideal cell has negligible internal resistance, so it would maintain a constant terminal voltage of
The voltage developed across a cell's terminals depends on the energy release of the chemical reactions of its electrodes and electrolyte. Alkaline and zinc–carbon cells have different chemistries, but approximately the same emf of 1.5 volts; likewise NiCd and NiMH cells have different chemistries, but approximately the same emf of 1.2 volts.[17] The high electrochemical potential changes in the reactions of lithium compounds give lithium cells emfs of 3 volts or more.[18]
Categories and types of batteries

From top to bottom: a large 4.5-volt (3R12) battery, a D Cell, a C cell, an AA cell, an AAA cell, an AAAA cell, an A23 battery, a 9-volt PP3 battery, and a pair of button cells (CR2032 and LR44).
Batteries are classified into primary and secondary forms.
- Primary batteries irreversibly transform chemical energy to electrical energy. When the supply of reactants is exhausted, energy cannot be readily restored to the battery.[19]
- Secondary batteries can be recharged; that is, they can have their chemical reactions reversed by supplying electrical energy to the cell, approximately restoring their original composition.[20]
Primary batteries
Primary batteries, or primary cells, can produce current immediately on assembly. These are most commonly used in portable devices that have low current drain, are used only intermittently, or are used well away from an alternative power source, such as in alarm and communication circuits where other electric power is only intermittently available. Disposable primary cells cannot be reliably recharged, since the chemical reactions are not easily reversible and active materials may not return to their original forms. Battery manufacturers recommend against attempting to recharge primary cells.[22]In general, these have higher energy densities than rechargeable batteries,[23] but disposable batteries do not fare well under high-drain applications with loads under 75 ohms (75 Ω).
Common types of disposable batteries include zinc–carbon batteries and alkaline batteries.
Secondary batteries
Secondary batteries, also known as secondary cells, or rechargeable batteries, must be charged before first use; they are usually assembled with active materials in the discharged state.
Rechargeable batteries are (re)charged by applying electric current, which reverses the chemical reactions that occur during discharge/use. Devices to supply the appropriate current are called chargers.
The oldest form of rechargeable battery is the lead–acid battery. This technology contains liquid electrolyte in an unsealed container, requiring that the battery be kept upright and the area be well ventilated to ensure safe dispersal of the hydrogen gas it produces during overcharging. The lead–acid battery is relatively heavy for the amount of electrical energy it can supply. Its low manufacturing cost and its high surge current levels make it common where its capacity (over approximately 10 Ah) is more important than weight and handling issues. A common application is the modern car battery, which can, in general, deliver a peak current of 450 amperes.
The sealed valve regulated lead–acid battery (VRLA battery) is popular in the automotive industry as a replacement for the lead–acid wet cell. The VRLA battery uses an immobilized sulfuric acid electrolyte, reducing the chance of leakage and extending shelf life.[24] VRLA batteries immobilize the electrolyte. The two types are:
Recent developments include batteries with embedded electronics such as USBCELL, which allows charging an AA battery through a USB connector,[25] nanoball batteries that allow for a discharge rate about 100x greater than current batteries, and smart battery packs with state-of-charge monitors and battery protection circuits that prevent damage on over-discharge. Low self-discharge (LSD) allows secondary cells to be charged prior to shipping.
Wet cells may be primary cells (non-rechargeable) or secondary cells (rechargeable). Originally, all practical primary batteries such as the Daniell cell were built as open-top glass jar wet cells. Other primary wet cells are the Leclanche cell, Grove cell, Bunsen cell, Chromic acid cell, Clark cell, and Weston cell. The Leclanche cell chemistry was adapted to the first dry cells. Wet cells are still used in automobile batteries and in industry for standby power for switchgear, telecommunication or large uninterruptible power supplies, but in many places batteries with gel cells have been used instead. These applications commonly use lead–acid or nickel–cadmium cells.

A dry cell uses a paste electrolyte, with only enough moisture to allow current to flow. Unlike a wet cell, a dry cell can operate in any orientation without spilling, as it contains no free liquid, making it suitable for portable equipment. By comparison, the first wet cells were typically fragile glass containers with lead rods hanging from the open top and needed careful handling to avoid spillage. Lead–acid batteries did not achieve the safety and portability of the dry cell until the development of the gel battery.
A common dry cell is the zinc–carbon battery, sometimes called the dry Leclanché cell, with a nominal voltage of 1.5 volts, the same as the alkaline battery (since both use the same zinc–manganese dioxide combination).
A standard dry cell comprises a zinc anode, usually in the form of a cylindrical pot, with a carbon cathode in the form of a central rod. The electrolyte is ammonium chloride in the form of a paste next to the zinc anode. The remaining space between the electrolyte and carbon cathode is taken up by a second paste consisting of ammonium chloride and manganese dioxide, the latter acting as a depolariser. In some designs, the ammonium chloride is replaced by zinc chloride.
A battery's capacity is the amount of electric charge it can deliver at the rated voltage. The more electrode material contained in the cell the greater its capacity. A small cell has less capacity than a larger cell with the same chemistry, although they develop the same open-circuit voltage.[27] Capacity is measured in units such as amp-hour (A·h).
The rated capacity of a battery is usually expressed as the product of 20 hours multiplied by the current that a new battery can consistently supply for 20 hours at 68 °F (20 °C), while remaining above a specified terminal voltage per cell. For example, a battery rated at 100 A·h can deliver 5 A over a 20-hour period at room temperature.
The fraction of the stored charge that a battery can deliver depends on multiple factors, including battery chemistry, the rate at which the charge is delivered (current), the required terminal voltage, the storage period, ambient temperature and other factors.[27]
The higher the discharge rate, the lower the capacity.[28] The relationship between current, discharge time and capacity for a lead acid battery is approximated (over a typical range of current values) by Peukert's law:
Internal energy losses and limitations on the rate that ions pass through the electrolyte cause battery efficiency to vary. Above a minimum threshold, discharging at a low rate delivers more of the battery's capacity than at a higher rate.
Installing batteries with varying A·h ratings does not affect device operation (although it may affect the operation interval) rated for a specific voltage unless load limits are exceeded. High-drain loads such as digital cameras can reduce total capacity, as happens with alkaline batteries. For example, a battery rated at 2000 mAh for a 10- or 20-hour discharge would not sustain a current of 1 A for a full two hours as its stated capacity implies.
4) battery technology was the fastest-charging/discharging, fully discharging in 10–20 seconds.[30]
As of 2013, the world's largest battery was in Hebei Province, China. It stored 36 megawatt-hours of electricity at a cost of $500 million.[31] Another large battery, composed of Ni–Cd cells, was in Fairbanks, Alaska. It covers 2,000 square metres (22,000 sq ft)—bigger than a football pitch—and weighs 1,300 tonnes, It was manufactured by ABB to provide backup power in the event of a blackout. The battery can provide 40 megawatts of power for up to seven minutes.[32] Sodium–sulfur batteries have been used to store wind power.[33] A 4.4 megawatt-hour battery system that can deliver 11 megawatts for 25 minutes stabilizes the output of the Auwahi wind farm in Hawaii.[34] Lithium–sulfur batteries were used on the longest and highest solar-powered flight.[35] The recharging speed of lithium-ion batteries can be increased by manufacturing changes.[36]
Old rechargeable batteries self-discharge more rapidly than disposable alkaline batteries, especially nickel-based batteries; a freshly charged nickel cadmium (NiCd) battery loses 10% of its charge in the first 24 hours, and thereafter discharges at a rate of about 10% a month. However, newer low self-discharge nickel metal hydride (NiMH) batteries and modern lithium designs display a lower self-discharge rate (but still higher than for primary batteries).
Most nickel-based batteries are partially discharged when purchased, and must be charged before first use.[38] Newer NiMH batteries are ready to be used when purchased, and have only 15% discharge in a year.[39]
Some deterioration occurs on each charge–discharge cycle. Degradation usually occurs because electrolyte migrates away from the electrodes or because active material detaches from the electrodes.
Low-capacity NiMH batteries (1700–2000 mA·h) can be charged some 1,000 times, whereas high-capacity NiMH batteries (above 2500 mA·h) last about 500 cycles.[40] NiCd batteries tend to be rated for 1,000 cycles before their internal resistance permanently increases beyond usable values.
"Deep-cycle" lead–acid batteries such as those used in electric golf carts have much thicker plates to extend longevity.[45] The main benefit of the lead–acid battery is its low cost; its main drawbacks are large size and weight for a given capacity and voltage.
Lead–acid batteries should never be discharged to below 20% of their capacity,[46] because internal resistance will cause heat and damage when they are recharged. Deep-cycle lead–acid systems often use a low-charge warning light or a low-charge power cut-off switch to prevent the type of damage that will shorten the battery's life.[47]
When a battery is recharged at an excessive rate, an explosive gas mixture of hydrogen and oxygen may be produced faster than it can escape from within the battery, leading to pressure build-up and eventual bursting of the battery case. In extreme cases, battery acid may spray violently from the casing and cause injury. Overcharging—that is, attempting to charge a battery beyond its electrical capacity—can also lead to a battery explosion, in addition to leakage or irreversible damage. It may also cause damage to the charger or device in which the overcharged battery is later used. In addition, disposing of a battery via incineration may cause an explosion as steam builds up within the sealed case.
Many battery chemicals are corrosive, poisonous or both. If leakage occurs, either spontaneously or through accident, the chemicals released may be dangerous.
For example, disposable batteries often use a zinc "can" both as a reactant and as the container to hold the other reagents. If this kind of battery is over-discharged, the reagents can emerge through the cardboard and plastic that form the remainder of the container. The active chemical leakage can then damage the equipment that the batteries power. For this reason, many electronic device manufacturers recommend removing the batteries from devices that will not be used for extended periods of time.
E-waste recycling services recover toxic substances, which can then be used for new batteries.[50]
Of the nearly three billion batteries purchased annually in the United States, about 179,000 tons end up in landfills across the country.[51]
In the United States, the Mercury-Containing and Rechargeable Battery Management Act of 1996 banned the sale of mercury-containing batteries, enacted uniform labeling requirements for rechargeable batteries and required that rechargeable batteries be easily removable.[52] California and New York City prohibit the disposal of rechargeable batteries in solid waste, and along with Maine require recycling of cell phones.[53] The rechargeable battery industry operates nationwide recycling programs in the United States and Canada, with dropoff points at local retailers.[53]
The Battery Directive of the European Union has similar requirements, in addition to requiring increased recycling of batteries and promoting research on improved battery recycling methods.[54]
In accordance with this directive all batteries to be sold within the EU must be marked with the "collection symbol" (A crossed-out wheeled bin). This must cover at least 3% of the surface of prismatic batteries and 1.5% of the surface of cylindrical batteries. All packaging must be marked likewise.[55]
Small button cells can be swallowed, in particular by young children. While in the digestive tract, the battery's electrical discharge may lead to tissue damage;[57] such damage is occasionally serious and can lead to death.
Ingested disk batteries do not usually cause problems unless they become lodged in the gastrointestinal tract. The most common place for disk batteries to become lodged is the esophagus, resulting in clinical sequelae. Batteries that successfully traverse the esophagus are unlikely to lodge elsewhere. The likelihood that a disk battery will lodge in the esophagus is a function of the patient's age and battery size. Disk batteries of 16 mm have become lodged in the esophagi of 2 children younger than 1 year.[citation needed] Older children do not have problems with batteries smaller than 21–23 mm. Liquefaction necrosis may occur because sodium hydroxide is generated by the current produced by the battery (usually at the anode). Perforation has occurred as rapidly as 6 hours after ingestion.[58]
A voltaic pile can be made from two coins (such as a nickel and a penny) and a piece of paper towel dipped in salt water. Such a pile generates a very low voltage but, when many are stacked in series, they can replace normal batteries for a short time.[63]
Sony has developed a biological battery that generates electricity from sugar in a way that is similar to the processes observed in living organisms. The battery generates electricity through the use of enzymes that break down carbohydrates.[64]
Lead acid cells can easily be manufactured at home, but a tedious charge/discharge cycle is needed to 'form' the plates. This is a process in which lead sulfate forms on the plates, and during charge is converted to lead dioxide (positive plate) and pure lead (negative plate). Repeating this process results in a microscopically rough surface, increasing the surface area. This increases the current the cell can deliver. For an example, see.[65]
Daniell cells are easy to make at home. Aluminium–air batteries can be produced with high-purity aluminium. Aluminium foil batteries will produce some electricity, but are not efficient, in part because a significant amount of (combustible) hydrogen gas is produced.
The oldest form of rechargeable battery is the lead–acid battery. This technology contains liquid electrolyte in an unsealed container, requiring that the battery be kept upright and the area be well ventilated to ensure safe dispersal of the hydrogen gas it produces during overcharging. The lead–acid battery is relatively heavy for the amount of electrical energy it can supply. Its low manufacturing cost and its high surge current levels make it common where its capacity (over approximately 10 Ah) is more important than weight and handling issues. A common application is the modern car battery, which can, in general, deliver a peak current of 450 amperes.
The sealed valve regulated lead–acid battery (VRLA battery) is popular in the automotive industry as a replacement for the lead–acid wet cell. The VRLA battery uses an immobilized sulfuric acid electrolyte, reducing the chance of leakage and extending shelf life.[24] VRLA batteries immobilize the electrolyte. The two types are:
- Gel batteries (or "gel cell") use a semi-solid electrolyte.
- Absorbed Glass Mat (AGM) batteries absorb the electrolyte in a special fiberglass matting.
Recent developments include batteries with embedded electronics such as USBCELL, which allows charging an AA battery through a USB connector,[25] nanoball batteries that allow for a discharge rate about 100x greater than current batteries, and smart battery packs with state-of-charge monitors and battery protection circuits that prevent damage on over-discharge. Low self-discharge (LSD) allows secondary cells to be charged prior to shipping.
Battery cell types
Many types of electrochemical cells have been produced, with varying chemical processes and designs, including galvanic cells, electrolytic cells, fuel cells, flow cells and voltaic piles.[26]Wet cell
A wet cell battery has a liquid electrolyte. Other names are flooded cell, since the liquid covers all internal parts, or vented cell, since gases produced during operation can escape to the air. Wet cells were a precursor to dry cells and are commonly used as a learning tool for electrochemistry. They can be built with common laboratory supplies, such as beakers, for demonstrations of how electrochemical cells work. A particular type of wet cell known as a concentration cell is important in understanding corrosion.Wet cells may be primary cells (non-rechargeable) or secondary cells (rechargeable). Originally, all practical primary batteries such as the Daniell cell were built as open-top glass jar wet cells. Other primary wet cells are the Leclanche cell, Grove cell, Bunsen cell, Chromic acid cell, Clark cell, and Weston cell. The Leclanche cell chemistry was adapted to the first dry cells. Wet cells are still used in automobile batteries and in industry for standby power for switchgear, telecommunication or large uninterruptible power supplies, but in many places batteries with gel cells have been used instead. These applications commonly use lead–acid or nickel–cadmium cells.
Dry cell

A dry cell uses a paste electrolyte, with only enough moisture to allow current to flow. Unlike a wet cell, a dry cell can operate in any orientation without spilling, as it contains no free liquid, making it suitable for portable equipment. By comparison, the first wet cells were typically fragile glass containers with lead rods hanging from the open top and needed careful handling to avoid spillage. Lead–acid batteries did not achieve the safety and portability of the dry cell until the development of the gel battery.
A common dry cell is the zinc–carbon battery, sometimes called the dry Leclanché cell, with a nominal voltage of 1.5 volts, the same as the alkaline battery (since both use the same zinc–manganese dioxide combination).
A standard dry cell comprises a zinc anode, usually in the form of a cylindrical pot, with a carbon cathode in the form of a central rod. The electrolyte is ammonium chloride in the form of a paste next to the zinc anode. The remaining space between the electrolyte and carbon cathode is taken up by a second paste consisting of ammonium chloride and manganese dioxide, the latter acting as a depolariser. In some designs, the ammonium chloride is replaced by zinc chloride.
Molten salt
Molten salt batteries are primary or secondary batteries that use a molten salt as electrolyte. They operate at high temperatures and must be well insulated to retain heat.Reserve
A reserve battery can be stored unassembled (unactivated and supplying no power) for a long period (perhaps years). When the battery is needed, then it is assembled (e.g., by adding electrolyte); once assembled, the battery is charged and ready to work. For example, a battery for an electronic artillery fuze might be activated by the impact of firing a gun: The acceleration breaks a capsule of electrolyte that activates the battery and powers the fuze's circuits. Reserve batteries are usually designed for a short service life (seconds or minutes) after long storage (years). A water-activated battery for oceanographic instruments or military applications becomes activated on immersion in water.Battery cell performance
A battery's characteristics may vary over load cycle, over charge cycle, and over lifetime due to many factors including internal chemistry, current drain, and temperature.Capacity and discharge
A battery's capacity is the amount of electric charge it can deliver at the rated voltage. The more electrode material contained in the cell the greater its capacity. A small cell has less capacity than a larger cell with the same chemistry, although they develop the same open-circuit voltage.[27] Capacity is measured in units such as amp-hour (A·h).
The rated capacity of a battery is usually expressed as the product of 20 hours multiplied by the current that a new battery can consistently supply for 20 hours at 68 °F (20 °C), while remaining above a specified terminal voltage per cell. For example, a battery rated at 100 A·h can deliver 5 A over a 20-hour period at room temperature.
The fraction of the stored charge that a battery can deliver depends on multiple factors, including battery chemistry, the rate at which the charge is delivered (current), the required terminal voltage, the storage period, ambient temperature and other factors.[27]
The higher the discharge rate, the lower the capacity.[28] The relationship between current, discharge time and capacity for a lead acid battery is approximated (over a typical range of current values) by Peukert's law:
t=QPIk
QP is the capacity when discharged at a rate of 1 amp.I is the current drawn from battery (A).t is the amount of time (in hours) that a battery can sustain.k is a constant around 1.3.
Internal energy losses and limitations on the rate that ions pass through the electrolyte cause battery efficiency to vary. Above a minimum threshold, discharging at a low rate delivers more of the battery's capacity than at a higher rate.
Installing batteries with varying A·h ratings does not affect device operation (although it may affect the operation interval) rated for a specific voltage unless load limits are exceeded. High-drain loads such as digital cameras can reduce total capacity, as happens with alkaline batteries. For example, a battery rated at 2000 mAh for a 10- or 20-hour discharge would not sustain a current of 1 A for a full two hours as its stated capacity implies.
C rate
The C-rate is a measure of the rate at which a battery is being discharged. It is defined as the discharge current divided by the theoretical current draw under which the battery would deliver its rated capacity in one hour. [29] A 1C discharge rate would deliver the battery's rated capacity in 1 hour. A 2C discharge rate means it will discharge twice as fast (30 minutes). A 1C discharge rate on a 1.6 Ah battery means a discharge current of 1.6 A. A 2C rate would mean a discharge current of 3.2 A. Standards for rechargeable batteries generally rate the capacity over a 4 hour, 8 hour or longer discharge time. Because of internal resistance loss and the chemical processes inside the cells, a battery rarely delivers nameplate rated capacity in only one hour. Types intended for special purposes, such as in a computer uninterruptible power supply, may be rated by manufacturers for discharge periods much less than one hour.Fast-charging, large and light batteries
As of 2012[update] Lithium iron phosphate (LiFePO4) battery technology was the fastest-charging/discharging, fully discharging in 10–20 seconds.[30]
As of 2013, the world's largest battery was in Hebei Province, China. It stored 36 megawatt-hours of electricity at a cost of $500 million.[31] Another large battery, composed of Ni–Cd cells, was in Fairbanks, Alaska. It covers 2,000 square metres (22,000 sq ft)—bigger than a football pitch—and weighs 1,300 tonnes, It was manufactured by ABB to provide backup power in the event of a blackout. The battery can provide 40 megawatts of power for up to seven minutes.[32] Sodium–sulfur batteries have been used to store wind power.[33] A 4.4 megawatt-hour battery system that can deliver 11 megawatts for 25 minutes stabilizes the output of the Auwahi wind farm in Hawaii.[34] Lithium–sulfur batteries were used on the longest and highest solar-powered flight.[35] The recharging speed of lithium-ion batteries can be increased by manufacturing changes.[36]
Battery lifetime
Available capacity of all batteries drops with decreasing temperature. In contrast to most of today's batteries, the Zamboni pile, invented in 1812, offers a very long service life without refurbishment or recharge, although it supplies current only in the nanoamp range. The Oxford Electric Bell has been ringing almost continuously since 1840 on its original pair of batteries, thought to be Zamboni piles.Self-discharge
Disposable batteries typically lose 8 to 20 percent of their original charge per year when stored at room temperature (20°–30 °C).[37] This is known as the "self-discharge" rate, and is due to non-current-producing "side" chemical reactions that occur within the cell even when no load is applied. The rate of side reactions is reduced for batteries are stored at lower temperatures, although some can be damaged by freezing.Old rechargeable batteries self-discharge more rapidly than disposable alkaline batteries, especially nickel-based batteries; a freshly charged nickel cadmium (NiCd) battery loses 10% of its charge in the first 24 hours, and thereafter discharges at a rate of about 10% a month. However, newer low self-discharge nickel metal hydride (NiMH) batteries and modern lithium designs display a lower self-discharge rate (but still higher than for primary batteries).
Corrosion
Internal parts may corrode and fail, or the active materials may be slowly converted to inactive forms.Physical component changes
The active material on the battery plates changes chemical composition on each charge and discharge cycle, active material may be lost due to physical changes of volume; further limiting the number of times the battery can be recharged.Most nickel-based batteries are partially discharged when purchased, and must be charged before first use.[38] Newer NiMH batteries are ready to be used when purchased, and have only 15% discharge in a year.[39]
Some deterioration occurs on each charge–discharge cycle. Degradation usually occurs because electrolyte migrates away from the electrodes or because active material detaches from the electrodes.
Low-capacity NiMH batteries (1700–2000 mA·h) can be charged some 1,000 times, whereas high-capacity NiMH batteries (above 2500 mA·h) last about 500 cycles.[40] NiCd batteries tend to be rated for 1,000 cycles before their internal resistance permanently increases beyond usable values.
Charge/discharge speed
Fast charging increases component changes, shortening battery lifespan.[40]Overcharging
If a charger cannot detect when the battery is fully charged then overcharging is likely, damaging it.[41]Memory effect
NiCd cells, if used in a particular repetitive manner, may show a decrease in capacity called "memory effect".[42] The effect can be avoided with simple practices. NiMH cells, although similar in chemistry, suffer less from memory effect.[43]Environmental conditions
Automotive lead–acid rechargeable batteries must endure stress due to vibration, shock, and temperature range. Because of these stresses and sulfation of their lead plates, few automotive batteries last beyond six years of regular use.[44] Automotive starting (SLI: Starting, Lighting, Ignition) batteries have many thin plates to maximize current. In general, the thicker the plates the longer the life. They are typically discharged only slightly before recharge."Deep-cycle" lead–acid batteries such as those used in electric golf carts have much thicker plates to extend longevity.[45] The main benefit of the lead–acid battery is its low cost; its main drawbacks are large size and weight for a given capacity and voltage.
Lead–acid batteries should never be discharged to below 20% of their capacity,[46] because internal resistance will cause heat and damage when they are recharged. Deep-cycle lead–acid systems often use a low-charge warning light or a low-charge power cut-off switch to prevent the type of damage that will shorten the battery's life.[47]
Storage
Battery life can be extended by storing the batteries at a low temperature, as in a refrigerator or freezer, which slows the side reactions. Such storage can extend the life of alkaline batteries by about 5%; rechargeable batteries can hold their charge much longer, depending upon type.[48] To reach their maximum voltage, batteries must be returned to room temperature; discharging an alkaline battery at 250 mA at 0 °C is only half as efficient as at 20 °C.[23] Alkaline battery manufacturers such as Duracell do not recommend refrigerating batteries.[22]Battery sizes
Primary batteries readily available to consumers range from tiny button cells used for electric watches, to the No. 6 cell used for signal circuits or other long duration applications. Secondary cells are made in very large sizes; very large batteries can power a submarine or stabilize an electrical grid and help level out peak loads.Hazards
Explosion
A battery explosion is caused by misuse or malfunction, such as attempting to recharge a primary (non-rechargeable) battery, or a short circuit. Car batteries are most likely to explode when a short-circuit generates very large currents. Car batteries produce hydrogen, which is very explosive, when they are overcharged (because of electrolysis of the water in the electrolyte). The amount of overcharging is usually very small and generates little hydrogen, which dissipates quickly. However, when "jumping" a car battery, the high current can cause the rapid release of large volumes of hydrogen, which can be ignited explosively by a nearby spark, for example, when disconnecting a jumper cable.When a battery is recharged at an excessive rate, an explosive gas mixture of hydrogen and oxygen may be produced faster than it can escape from within the battery, leading to pressure build-up and eventual bursting of the battery case. In extreme cases, battery acid may spray violently from the casing and cause injury. Overcharging—that is, attempting to charge a battery beyond its electrical capacity—can also lead to a battery explosion, in addition to leakage or irreversible damage. It may also cause damage to the charger or device in which the overcharged battery is later used. In addition, disposing of a battery via incineration may cause an explosion as steam builds up within the sealed case.
Leakage
Many battery chemicals are corrosive, poisonous or both. If leakage occurs, either spontaneously or through accident, the chemicals released may be dangerous.
For example, disposable batteries often use a zinc "can" both as a reactant and as the container to hold the other reagents. If this kind of battery is over-discharged, the reagents can emerge through the cardboard and plastic that form the remainder of the container. The active chemical leakage can then damage the equipment that the batteries power. For this reason, many electronic device manufacturers recommend removing the batteries from devices that will not be used for extended periods of time.
Toxic materials
Many types of batteries employ toxic materials such as lead, mercury, and cadmium as an electrode or electrolyte. When each battery reaches end of life it must be disposed of to prevent environmental damage.[49] Battery are one form of electronic waste (e-waste).E-waste recycling services recover toxic substances, which can then be used for new batteries.[50]
Of the nearly three billion batteries purchased annually in the United States, about 179,000 tons end up in landfills across the country.[51]
In the United States, the Mercury-Containing and Rechargeable Battery Management Act of 1996 banned the sale of mercury-containing batteries, enacted uniform labeling requirements for rechargeable batteries and required that rechargeable batteries be easily removable.[52] California and New York City prohibit the disposal of rechargeable batteries in solid waste, and along with Maine require recycling of cell phones.[53] The rechargeable battery industry operates nationwide recycling programs in the United States and Canada, with dropoff points at local retailers.[53]
The Battery Directive of the European Union has similar requirements, in addition to requiring increased recycling of batteries and promoting research on improved battery recycling methods.[54]
In accordance with this directive all batteries to be sold within the EU must be marked with the "collection symbol" (A crossed-out wheeled bin). This must cover at least 3% of the surface of prismatic batteries and 1.5% of the surface of cylindrical batteries. All packaging must be marked likewise.[55]
Ingestion
Batteries may be harmful or fatal if swallowed.[56]Small button cells can be swallowed, in particular by young children. While in the digestive tract, the battery's electrical discharge may lead to tissue damage;[57] such damage is occasionally serious and can lead to death.
Ingested disk batteries do not usually cause problems unless they become lodged in the gastrointestinal tract. The most common place for disk batteries to become lodged is the esophagus, resulting in clinical sequelae. Batteries that successfully traverse the esophagus are unlikely to lodge elsewhere. The likelihood that a disk battery will lodge in the esophagus is a function of the patient's age and battery size. Disk batteries of 16 mm have become lodged in the esophagi of 2 children younger than 1 year.[citation needed] Older children do not have problems with batteries smaller than 21–23 mm. Liquefaction necrosis may occur because sodium hydroxide is generated by the current produced by the battery (usually at the anode). Perforation has occurred as rapidly as 6 hours after ingestion.[58]
Battery chemistry
Primary batteries and their characteristics
| Chemistry | Anode (-) | Cathode (+) | Maximum Voltage (Theoretical) (V) |
Working Voltage (Practical) (V) |
Specific energy [MJ/kg] | Elaboration | Shelf Life at 25 °C (80% Capacity) (Months) |
|---|---|---|---|---|---|---|---|
| Zinc–carbon | Zn | MnO2 | 1.6 | 1.2 | 0.13 | Inexpensive. | 18 |
| Zinc–chloride | 1.5 | Also known as "heavy-duty", inexpensive. | |||||
| Alkaline (zinc–manganese dioxide) |
Zn | MnO2 | 1.5 | 1.15 | 0.4–0.59 | Moderate energy density. Good for high- and low-drain uses. |
30 |
| Nickel oxyhydroxide (zinc–manganese dioxide/nickel oxyhydroxide) |
1.7 | Moderate energy density. Good for high drain uses. |
|||||
| Lithium (lithium–copper oxide) Li–CuO |
1.7 | No longer manufactured. Replaced by silver oxide (IEC-type "SR") batteries. |
|||||
| Lithium (lithium–iron disulfide) LiFeS2 |
1.5 | Expensive. Used in 'plus' or 'extra' batteries. |
|||||
| Lithium (lithium–manganese dioxide) LiMnO2 |
3.0 | 0.83–1.01 | Expensive. Used only in high-drain devices or for long shelf-life due to very low rate of self-discharge. 'Lithium' alone usually refers to this type of chemistry. |
||||
| Lithium (lithium–carbon fluoride) Li–(CF)n |
Li | (CF)n | 3.6 | 3.0 | 120 | ||
| Lithium (lithium–chromium oxide) Li–CrO2 |
Li | CrO2 | 3.8 | 3.0 | 108 | ||
| Mercury oxide | Zn | HgO | 1.34 | 1.2 | High-drain and constant voltage. Banned in most countries because of health concerns. |
36 | |
| Zinc–air | Zn | O2 | 1.6 | 1.1 | 1.59[59] | Used mostly in hearing aids. | |
| Zamboni pile | Zn | Ag or Au | 0.8 | Very long life Very low (nanoamp) current |
>2000 | ||
| Silver-oxide (silver–zinc) | Zn | Ag2O | 1.85 | 1.5 | 0.47 | Very expensive. Used only commercially in 'button' cells. |
30 |
| Magnesium | Mg | MnO2 | 2.0 | 1.5 | 40 |
Secondary (rechargeable) batteries and their characteristics
| Chemistry | Cell Voltage |
Specific energy [MJ/kg] |
Comments |
|---|---|---|---|
| NiCd | 1.2 | 0.14 | Inexpensive. High-/low-drain, moderate energy density. Can withstand very high discharge rates with virtually no loss of capacity. Moderate rate of self-discharge. Environmental hazard due to Cadmium – use now virtually prohibited in Europe. |
| Lead–acid | 2.1 | 0.14 | Moderately expensive. Moderate energy density. Moderate rate of self-discharge. Higher discharge rates result in considerable loss of capacity. Environmental hazard due to Lead. Common use – Automobile batteries |
| NiMH | 1.2 | 0.36 | Inexpensive. Performs better than alkaline batteries in higher drain devices. Traditional chemistry has high energy density, but also a high rate of self-discharge. Newer chemistry has low self-discharge rate, but also a ~25% lower energy density. Used in some cars. |
| NiZn | 1.6 | 0.36 | Moderately inexpensive. High drain device suitable. Low self-discharge rate. Voltage closer to alkaline primary cells than other secondary cells. No toxic components. Newly introduced to the market (2009). Has not yet established a track record. Limited size availability. |
| AgZn | 1.86 1.5 |
0.46 | Smaller volume than equivalent Li-ion. Extremely expensive due to silver. Very high energy density. Very high drain capable. For many years considered obsolete due to high silver prices. Cell suffers from oxidation if unused. Reactions are not fully understood. Terminal voltage very stable but suddenly drops to 1.5 volts at 70–80% charge (believed to be due to presence of both argentous and argentic oxide in positive plate – one is consumed first). Has been used in lieu of primary battery (moon buggy). Is being developed once again as a replacement for Li-ion. |
| Lithium ion | 3.6 | 0.46 | Very expensive. Very high energy density. Not usually available in "common" battery sizes. Very common in laptop computers, moderate to high-end digital cameras, camcorders, and cellphones. Very low rate of self-discharge. Terminal voltage unstable (varies from 4.2 to 3.0 volts during discharge). Volatile: Chance of explosion if short-circuited, allowed to overheat, or not manufactured with rigorous quality standards. |
Homemade cells
Almost any liquid or moist object that has enough ions to be electrically conductive can serve as the electrolyte for a cell. As a novelty or science demonstration, it is possible to insert two electrodes made of different metals into a lemon,[60] potato,[61] etc. and generate small amounts of electricity. "Two-potato clocks" are also widely available in hobby and toy stores; they consist of a pair of cells, each consisting of a potato (lemon, et cetera) with two electrodes inserted into it, wired in series to form a battery with enough voltage to power a digital clock.[62] Homemade cells of this kind are of no practical use.A voltaic pile can be made from two coins (such as a nickel and a penny) and a piece of paper towel dipped in salt water. Such a pile generates a very low voltage but, when many are stacked in series, they can replace normal batteries for a short time.[63]
Sony has developed a biological battery that generates electricity from sugar in a way that is similar to the processes observed in living organisms. The battery generates electricity through the use of enzymes that break down carbohydrates.[64]
Lead acid cells can easily be manufactured at home, but a tedious charge/discharge cycle is needed to 'form' the plates. This is a process in which lead sulfate forms on the plates, and during charge is converted to lead dioxide (positive plate) and pure lead (negative plate). Repeating this process results in a microscopically rough surface, increasing the surface area. This increases the current the cell can deliver. For an example, see.[65]
Daniell cells are easy to make at home. Aluminium–air batteries can be produced with high-purity aluminium. Aluminium foil batteries will produce some electricity, but are not efficient, in part because a significant amount of (combustible) hydrogen gas is produced.






