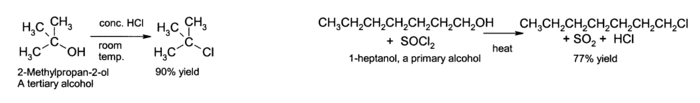Ball-and-stick model of the hydroxyl (-OH) functional group in an alcohol molecule (R
3COH). The three "R's" stand for carbon substituents or hydrogen atoms.
[1]

The hydroxyl (-OH) functional group with bond angle
In
chemistry, an
alcohol is any
organic compound in which the
hydroxyl functional group (-
O H) is bound to a
saturated carbon atom.
[2] The term alcohol originally referred to the primary alcohol
ethyl alcohol (ethanol), the predominant alcohol in
alcoholic beverages.
Muhammad ibn Zakariya al-Razi first discovered alcohol (ethanol) in its pure form in
Persia.
The suffix
-ol appears in the
IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix
hydroxy- will appear in the
IUPAC name. The suffix
-ol in non-systematic names (such as
paracetamol or
cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples
glucose and
sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols are the simple
acyclic alcohols, the general formula for which is C
nH
2n+1OH.
Occurrence in nature
Alcohols have been found outside the
Solar System where they can be found in low densities in star and planetary-system-forming regions of space.
[3][non-primary source needed]
Toxicity
Ethanol is thought to cause harm partly as a result of direct damage to DNA caused by its metabolites.
[7]
Ethanol's toxicity is largely caused by its primary metabolite,
acetaldehyde (systematically ethanal)
[8][9] and secondary metabolite,
acetic acid.
[9][10][11][12] All primary alcohols are broken down into aldehydes then to carboxylic acids whose toxicities are similar to acetaldehyde and acetic acid.
[citation needed] Metabolite toxicity is reduced in rats fed
N-acetylcysteine[8][13] and
thiamine.
[14]
Tertiary alcohols cannot be metabolized into aldehydes
[15] and as a result they cause no hangover or toxicity through this mechanism.
Some
secondary and
tertiary alcohols are less poisonous than ethanol because the liver is unable to metabolize them into toxic by-products.
[16] This makes them more suitable for recreational and medicinal
[17] use as the chronic harms are lower.
[medical citation needed] Ethchlorvynol and
tert-amyl alcohol are tertiary alcohols which have seen both medicinal and recreational use.
[18]
Other alcohols are substantially more poisonous than ethanol, partly because they take much longer to be metabolized and partly because their metabolism produces substances that are even more toxic. Methanol (wood alcohol), for instance, is oxidized to
formaldehyde and then to the poisonous
formic acid in the liver by
alcohol dehydrogenase and
formaldehyde dehydrogenase enzymes, respectively; accumulation of formic acid can lead to blindness or death.
[19] Likewise, poisoning due to other alcohols such as
ethylene glycol or
diethylene glycol are due to their metabolites, which are also produced by alcohol dehydrogenase.
[20][21]
Methanol itself, while poisonous (
LD50 5628 mg/kg, oral, rat
[22]), has a much weaker
sedative effect than ethanol.
Isopropyl alcohol is oxidized to form acetone by
alcohol dehydrogenase in the liver but has occasionally been abused by
alcoholics, leading to a range of adverse health effects.
[23][better source needed][24][better source needed]
Treatment
An effective treatment to prevent toxicity after methanol or ethylene glycol ingestion is to administer ethanol. Alcohol dehydrogenase has a higher affinity for ethanol, thus preventing methanol from binding and acting as a
substrate. Any remaining methanol will then have time to be excreted through the kidneys.
[19][25][26]
Nomenclature
Systematic names
IUPAC nomenclature is used in scientific publications and where precise identification of the substance is important, especially in cases where the relative complexity of the molecule does not make such a systematic name unwieldy. In the IUPAC system, in naming simple alcohols, the name of the alkane chain loses the terminal "e" and adds "ol", e.g., as in "methanol" and "ethanol".
[27] When necessary, the position of the hydroxyl group is indicated by a number between the alkane name and the "ol":
propan-1-ol for CH
3CH
2CH
2OH,
propan-2-ol for CH
3CH(OH)CH
3. If a higher priority group is present (such as an
aldehyde,
ketone, or
carboxylic acid), then the prefix "hydroxy" is used,
[27] e.g., as in 1-hydroxy-2-propanone (CH
3C(O)CH
2OH).
[28]
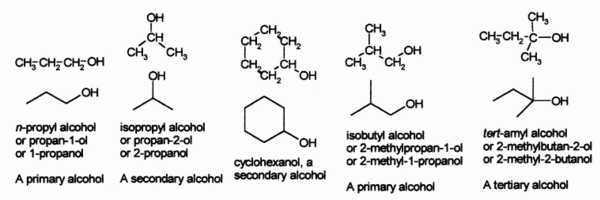
Some examples of simple alcohols and how to name them
Common names
In other less formal contexts, an alcohol is often called with the name of the corresponding alkyl group followed by the word "alcohol", e.g.,
methyl alcohol,
ethyl alcohol.
Propyl alcohol may be
n-propyl alcohol or
isopropyl alcohol, depending on whether the hydroxyl group is bonded to the end or middle carbon on the straight
propane chain. As described under systematic naming, if another group on the molecule takes priority, the alcohol moiety is often indicated using the "hydroxy-" prefix.
Alcohols are then classified into primary, secondary (
sec-,
s-), and tertiary (
tert-,
t-), based upon the number of carbon atoms connected to the carbon atom that bears the
hydroxyl functional group. (The respective numeric shorthands 1°, 2°, and 3° are also sometimes used in informal settings.
[citation needed]) The primary alcohols have general formulas RCH
2OH; methanol (CH
3OH is the simplest primary alcohol (R=H), and after it, ethanol (R=CH
3). Secondary alcohols can be referred to with the shorthand RR'CHOH; 2-propanol is the simplest example (R=R'=CH
3). Tertiary alcohols can be referred to with the shorthand RR'R"COH;
tert-butanol (2-methylpropan-2-ol) is the simplest example (R=R'=R"=CH
3). In these shorthands, R, R', and R" represent
substituents, alkyl or other attached, generally organic groups.
| Chemical Formula |
IUPAC Name |
Common Name |
| Monohydric alcohols |
| CH3OH |
Methanol |
Wood alcohol |
| C2H5OH |
Ethanol |
Alcohol |
| C3H7OH |
Isopropyl alcohol |
Rubbing alcohol |
| C4H9OH |
Butyl alcohol |
Butanol |
| C5H11OH |
Pentanol |
Amyl alcohol |
| C16H33OH |
Hexadecan-1-ol |
Cetyl alcohol |
| Polyhydric alcohols |
| C2H4(OH)2 |
Ethane-1,2-diol |
Ethylene glycol |
| C3H6(OH)2 |
Propane-1,2-diol |
Propylene Glycol |
| C3H5(OH)3 |
Propane-1,2,3-triol |
Glycerol |
| C4H6(OH)4 |
Butane-1,2,3,4-tetraol |
Erythritol, Threitol |
| C5H7(OH)5 |
Pentane-1,2,3,4,5-pentol |
Xylitol |
| C6H8(OH)6 |
Hexane-1,2,3,4,5,6-hexol |
Mannitol, Sorbitol |
| C7H9(OH)7 |
Heptane-1,2,3,4,5,6,7-heptol |
Volemitol |
| Unsaturated aliphatic alcohols |
| C3H5OH |
Prop-2-ene-1-ol |
Allyl alcohol |
| C10H17OH |
3,7-Dimethylocta-2,6-dien-1-ol |
Geraniol |
| C3H3OH |
Prop-2-in-1-ol |
Propargyl alcohol |
| Alicyclic alcohols |
| C6H6(OH)6 |
Cyclohexane-1,2,3,4,5,6-hexol |
Inositol |
| C10H19OH |
2 - (2-propyl)-5-methyl-cyclohexane-1-ol |
Menthol |
Alkyl chain variations in alcohols
Short-chain alcohols have alkyl chains of 1-3 carbons. Medium-chain alcohols have alkyl chains of 4-7 carbons. Long-chain alcohols (also known as
fatty alcohols) have alkyl chains of 8-21 carbons, and very long-chain alcohols have alkyl chains of 22 carbons or longer.
[29]
Simple alcohols
"Simple alcohols" appears to be a completely undefined term. However, simple alcohols are often referred to by common names derived by adding the word "alcohol" to the name of the appropriate alkyl group. For instance, a chain consisting of one carbon (a methyl group, CH
3) with an OH group attached to the carbon is called "methyl alcohol" while a chain of two carbons (an ethyl group, CH
2CH
3) with an OH group connected to the CH
2 is called "ethyl alcohol." For more complex alcohols, the IUPAC nomenclature must be used.
[30]
Simple alcohols, in particular ethanol and methanol, possess
denaturing and inert rendering properties, leading to their use as anti-microbial agents in medicine, pharmacy, and industry.
[citation needed]
Higher alcohols
Encyclopædia Britannica states, "The higher alcohols - those containing 4 to 10 carbon atoms – are somewhat viscous, or oily, and they have heavier fruity odours. Some of the highly branched alcohols and many alcohols containing more than 12 carbon atoms are solids at room temperature."
[31]
Like ethanol,
butanol can be produced by fermentation processes. Saccharomyces yeast are known to produce these higher alcohols at temperatures above 75 °F (24 °C). The bacterium
Clostridium acetobutylicum can feeds on
cellulose to produce butanol on an industrial scale.
Etymology
The word
alcohol appears in English as a term for a very fine powder in the sixteenth century. It was borrowed from French, which took it from medical
Latin.
Ultimately the word is from the
Arabic كحل (
al-kuḥl, "
kohl, a powder used as an eyeliner").
Al- is the Arabic
definitive article, equivalent to
the in English;
alcohol was originally used for the very fine powder produced by the
sublimation of the natural mineral
stibnite to form
antimony sulfide Sb2S3 (hence the essence or "spirit" of the substance), which was used as an
antiseptic, eyeliner, and cosmetic (see
kohl (cosmetics)).
Bartholomew Traheron, in his 1543 translation of
John of Vigo, introduces the word as a term used by "barbarous" (
Moorish) authors for "fine powder." Vigo wrote:
the barbarous auctours use alcohol, or (as I fynde it sometymes wryten) alcofoll, for moost fine poudre.
The 1657
Lexicon Chymicum by William Johnson glosses the word as
antimonium sive stibium. By extension, the word came to refer to any fluid obtained by distillation, including "alcohol of wine," the distilled essence of wine.
Libavius in
Alchymia (1594) refers to
vini alcohol vel vinum alcalisatum. Johnson (1657) glosses
alcohol vini as
quando omnis superfluitas vini a vino separatur, ita ut accensum ardeat donec totum consumatur, nihilque fæcum aut phlegmatis in fundo remaneat. The word's meaning became restricted to "spirit of wine" (the chemical known today as
ethanol) in the 18th century and was extended to the class of substances so-called as "alcohols" in modern chemistry after 1850.
The current Arabic name for alcohol (
ethanol) is الغول al-ġawl – properly meaning "spirit" or "demon" – with the sense "the thing that gives the wine its headiness" (in the Qur'an
sura 37 verse 47).
[32] The term
ethanol was invented 1838, modeled on the German word äthyl (Liebig), which is in turn based on Greek
aither ether and
hyle "stuff."
[33]
Physical and chemical properties
Alcohols have an odor that is often described as “biting” and as “hanging” in the nasal passages. Ethanol has a slightly sweeter (or more fruit-like) odor than the other alcohols.
In general, the
hydroxyl group makes the alcohol molecule
polar. Those groups can form
hydrogen bonds to one another and to other compounds (except in
certain large molecules where the hydroxyl is protected by
steric hindrance of adjacent groups
[34]). This hydrogen bonding means that alcohols can be used as
protic solvents. Two opposing solubility trends in alcohols are: the tendency of the polar OH to promote solubility in water, and the tendency of the carbon chain to resist it. Thus, methanol, ethanol, and propanol are
miscible in water because the hydroxyl group wins out over the short carbon chain.
Butanol, with a four-carbon chain, is moderately soluble because of a balance between the two trends. Alcohols of five or more carbons such as
pentanol and higher are effectively insoluble in water because of the hydrocarbon chain's dominance. All simple alcohols are miscible in organic solvents.
Because of
hydrogen bonding, alcohols tend to have higher boiling points than comparable
hydrocarbons and
ethers. The boiling point of the alcohol ethanol is 78.29 °C, compared to 69 °C for the hydrocarbon
hexane (a common constituent of
gasoline), and 34.6 °C for
diethyl ether.
Alcohols, like water, can show either acidic or basic properties at the -OH group. With a
pKa of around 16-19, they are, in general, slightly weaker
acids than
water, but they are still able to react with strong bases such as
sodium hydride or reactive metals such as
sodium. The
salts that result are called
alkoxides, with the general formula
RO
− M+.
Meanwhile, the oxygen atom has
lone pairs of nonbonded electrons that render it weakly
basic in the presence of strong acids such as
sulfuric acid. For example, with methanol:

Alcohols can also undergo
oxidation to give
aldehydes,
ketones, or
carboxylic acids, or they can be dehydrated to
alkenes. They can react to form
ester compounds, and they can (if activated first) undergo
nucleophilic substitution reactions. The lone pairs of electrons on the oxygen of the hydroxyl group also makes alcohols
nucleophiles. For more details, see the
reactions of alcohols section below.
As one moves from primary to secondary to tertiary alcohols with the same backbone, the hydrogen bond strength, the boiling point, and the acidity typically decrease.
Applications
Alcohol has a long history of several uses worldwide. It is found in alcoholic beverages sold to adults, as fuel, and also has many scientific, medical, and industrial uses. The term
alcohol-free is often used to describe a product that does not contain alcohol. Some consumers of some commercially prepared products may view alcohol as an undesirable ingredient, particularly in products intended for children.
Alcoholic beverages
Alcoholic beverages, typically containing 3–40%
ethanol by volume, have been produced and consumed by humans since pre-historic times. Other alcohols such as
2-methyl-2-butanol (found in
beer) and
γ-hydroxybutyric acid are also consumed by humans for their psychoactive effects.
Antifreeze
A 50%
v/v (by volume) solution of
ethylene glycol in water is commonly used as an
antifreeze.
Antiseptics
Ethanol can be used as an
antiseptic to disinfect the skin before injections are given, often along with
iodine. Ethanol-based
soaps are becoming common in restaurants and are convenient because they do not require drying due to the volatility of the compound. Alcohol based gels have become common as
hand sanitizers.
Fuels
Some alcohols, mainly
ethanol and
methanol, can be used as an
alcohol fuel. Fuel performance can be increased in
forced induction internal combustion engines by injecting alcohol into the air intake after the
turbocharger or
supercharger has pressurized the air. This cools the pressurized air, providing a denser air charge, which allows for more fuel, and therefore more power.
Preservative
Alcohol is often used as a
preservative for
specimens in the fields of science and medicine.
Solvents
Hydroxyl groups (-OH), found in alcohols, are
polar and therefore
hydrophilic (water loving) but their carbon chain portion is
non-polar which make them
hydrophobic. The molecule increasingly becomes overall more nonpolar and therefore less soluble in the polar water as the carbon chain becomes longer.
[36] Methanol has the shortest carbon chain of all alcohols (one carbon atom) followed by ethanol (two carbon atoms.)
Alcohols have applications in industry and science as reagents or
solvents. Because of its relatively low toxicity compared with other alcohols and ability to dissolve
non-polar substances, ethanol can be used as a solvent in medical drugs,
perfumes, and vegetable essences such as
vanilla. In
organic synthesis, alcohols serve as versatile intermediates.
Production
Ziegler and oxo processes
In the
Ziegler process, linear alcohols are produced from ethylene and
triethylaluminium followed by oxidation and hydrolysis.
[37] An idealized synthesis of
1-octanol is shown:
- Al(C2H5)3 + 9 C2H4 → Al(C8H17)3
- Al(C8H17)3 + 3 O + 3 H2O → 3 HOC8H17 + Al(OH)3
The process generates a range of alcohols that are separated by
distillation.
Many higher alcohols are produced by
hydroformylation of alkenes followed by hydrogenation. When applied to a terminal alkene, as is common, one typically obtains a linear alcohol:
[37]
- RCH=CH2 + H2 + CO → RCH2CH2CHO
- RCH2CH2CHO + 3 H2 → RCH2CH2CH2OH
Such processes give
fatty alcohols, which are useful for detergents.
Hydration reactions
Low molecular weight alcohols of industrial importance are produced by the addition of water to alkenes. Ethanol, isopropanol, 2-butanol, and tert-butanol are produced by this general method. Two implementations are employed, the direct and indirect methods. The direct method avoids the formation of stable intermediates, typically using acid catalysts. In the indirect method, the alkene is converted to the
sulfate ester, which is subsequently hydrolyzed. The direct
hydration using
ethylene (
ethylene hydration)
[38] or other alkenes from
cracking of fractions of distilled
crude oil.
Hydration is also used industrially to produce the diol
ethylene glycol from
ethylene oxide.
Biological routes
Ethanol is obtained by
fermentation using
glucose produced from sugar from the
hydrolysis of
starch, in the presence of yeast and temperature of less than 37 °C to produce ethanol. For instance, such a process might proceed by the conversion of
sucrose by the enzyme
invertase into
glucose and
fructose, then the conversion of
glucose by the enzyme
zymase into
ethanol (and carbon dioxide).
Several of the benign bacteria
[which?] in the intestine use
fermentation as a form of
anaerobic metabolism. This
metabolic reaction produces
ethanol as a waste product, just like
aerobic respiration produces
carbon dioxide and
water. Thus, human bodies contain some quantity of alcohol endogenously produced by these bacteria. In rare cases, this can be sufficient to cause "
auto-brewery syndrome" in which intoxicating quantities of alcohol are produced.
[39][40][41]
Laboratory synthesis
Several methods exist for the preparation of alcohols in the laboratory.
Substitution
Primary
alkyl halides react with aqueous
NaOH or
KOH mainly to primary alcohols in
nucleophilic aliphatic substitution. (Secondary and especially tertiary alkyl halides will give the elimination (alkene) product instead).
Grignard reagents react with
carbonyl groups to secondary and tertiary alcohols. Related reactions are the
Barbier reaction and the
Nozaki-Hiyama reaction.
Reduction
Aldehydes or
ketones are
reduced with
sodium borohydride or
lithium aluminium hydride (after an acidic workup). Another reduction by aluminiumisopropylates is the
Meerwein-Ponndorf-Verley reduction.
Noyori asymmetric hydrogenation is the asymmetric reduction of β-keto-esters.
Hydrolysis
Alkenes engage in an acid catalysed
hydration reaction using concentrated sulfuric acid as a catalyst that gives usually secondary or tertiary alcohols. The
hydroboration-oxidation and
oxymercuration-reduction of alkenes are more reliable in organic synthesis. Alkenes react with NBS and water in
halohydrin formation reaction.
Amines can be converted to
diazonium salts, which are then hydrolyzed.
The formation of a secondary alcohol via reduction and hydration is shown:

Reactions
Deprotonation
Alcohols can behave as weak acids, undergoing
deprotonation. The deprotonation reaction to produce an
alkoxide salt is performed either with a strong base such as sodium hydride or
n-butyllithium or with sodium or potassium metal.
- 2 R-OH + 2 NaH → 2 R-O−Na+ + 2H2↑
- 2 R-OH + 2 Na → 2 R-O−Na+ + H2
- 2 CH3CH2-OH + 2 Na → 2 CH3-CH2-O−Na+ + H2↑
Water is similar in
pKa to many alcohols, so with
sodium hydroxide there is an
equilibrium set-up, which usually lies to the left:
- R-OH + NaOH ⇌ R-O−Na+ + H2O (equilibrium to the left)
It should be noted, however, that the bases used to deprotonate alcohols are strong themselves. The bases used and the alkoxides created are both highly moisture-sensitive chemical reagents.
The acidity of alcohols is also affected by the overall stability of the alkoxide ion.
Electron-withdrawing groups attached to the carbon containing the hydroxyl group will serve to stabilize the alkoxide when formed, thus resulting in greater acidity. On the other hand, the presence of
electron-donating group will result in a less stable alkoxide ion formed. This will result in a scenario whereby the unstable alkoxide ion formed will tend to accept a proton to reform the original alcohol.
With
alkyl halides alkoxides give rise to
ethers in the
Williamson ether synthesis.
Nucleophilic substitution
The OH group is not a good
leaving group in
nucleophilic substitution reactions, so neutral alcohols do not react in such reactions. However, if the oxygen is first protonated to give R−OH
2+, the leaving group (
water) is much more stable, and the nucleophilic substitution can take place. For instance, tertiary alcohols react with
hydrochloric acid to produce tertiary
alkyl halides, where the
hydroxyl group is replaced by a
chlorine atom by
unimolecular nucleophilic substitution. If primary or secondary alcohols are to be reacted with
hydrochloric acid, an activator such as
zinc chloride is needed. In alternative fashion, the conversion may be performed directly using
thionyl chloride.
[1]
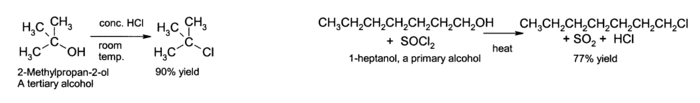
Alcohols may, likewise, be converted to alkyl bromides using
hydrobromic acid or
phosphorus tribromide, for example:
- 3 R-OH + PBr3 → 3 RBr + H3PO3
In the
Barton-McCombie deoxygenation an alcohol is deoxygenated to an
alkane with
tributyltin hydride or a
trimethylborane-water complex in a
radical substitution reaction.
Dehydration
Alcohols are themselves nucleophilic, so R−OH
2+ can react with ROH to produce
ethers and water in a
dehydration reaction, although this reaction is rarely used except in the manufacture of
diethyl ether.
More useful is the E1
elimination reaction of alcohols to produce
alkenes. The reaction, in general, obeys
Zaitsev's Rule, which states that the most stable (usually the most substituted) alkene is formed. Tertiary alcohols eliminate easily at just above room temperature, but primary alcohols require a higher temperature.
This is a diagram of acid catalysed dehydration of ethanol to produce
ethene:

A more controlled elimination reaction is the
Chugaev elimination with
carbon disulfide and
iodomethane.
Esterification
To form an
ester from an alcohol and a
carboxylic acid the reaction, known as
Fischer esterification, is usually performed at
reflux with a
catalyst of concentrated sulfuric acid:
- R-OH + R'-COOH → R'-COOR + H2O
In order to drive the equilibrium to the right and produce a good
yield of ester, water is usually removed, either by an excess of H
2SO
4 or by using a
Dean-Stark apparatus. Esters may also be prepared by reaction of the alcohol with an
acid chloride in the presence of a base such as
pyridine.
Other types of ester are prepared in a similar manner – for example,
tosyl (tosylate) esters are made by reaction of the alcohol with p-
toluenesulfonyl chloride in pyridine.
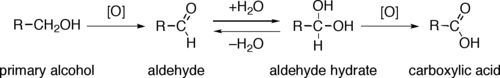
Oxidation
Primary alcohols (R-CH
2-OH) can be oxidized either to
aldehydes (R-CHO) or to
carboxylic acids (R-CO
2H), while the oxidation of secondary alcohols (R
1R
2CH-OH) normally terminates at the
ketone (R
1R
2C=O) stage. Tertiary alcohols (R
1R
2R
3C-OH) are resistant to oxidation.
The direct
oxidation of primary alcohols to carboxylic acids normally proceeds via the corresponding aldehyde, which is transformed via an
aldehyde hydrate (R-CH(OH)
2) by reaction with water before it can be further oxidized to the carboxylic acid.
Reagents useful for the transformation of primary alcohols to aldehydes are normally also suitable for the
oxidation of secondary alcohols to ketones. These include
Collins reagent and
Dess-Martin periodinane. The direct oxidation of primary alcohols to carboxylic acids can be carried out using
potassium permanganate or the
Jones reagent.