| Vitamin B12 deficiency | |
|---|---|
| Other names | Hypocobalaminemia, cobalamin deficiency |
 | |
| Cyanocobalamin | |
| Specialty | Neurology, hematology |
| Symptoms | Decreased ability to think, depression, irritability, abnormal sensations, changes in reflexes |
| Complications | Megaloblastic anemia |
| Causes | Poor absorption, decreased intake, increased requirements |
| Diagnostic method | Blood levels below 120–180 picomol/L (170–250 pg/mL) in adults |
| Prevention | Supplementation in those at high risk |
| Treatment | Supplementation by mouth or injection |
| Frequency | 6% (<60 20="" old="" years="">60 years old) |
Vitamin B12 deficiency, also known as cobalamin deficiency, is the medical condition of low blood levels of vitamin B12. In mild deficiency a person may feel tired and have a reduced number of red blood cells (anemia). In moderate deficiency there may be inflammation of the tongue and the beginning of neurological problems including abnormal sensations such as pins and needles, while severe deficiency may include reduced heart function and greater neurological problems.
Neurological problems may include changes in reflexes, poor muscle function, memory problems, decreased taste, and in extreme cases psychosis. Sometimes temporary infertility may also occur. In young children symptoms include poor growth, poor development, and difficulties with movement. Without early treatment, some of the changes may be permanent.
Common causes include poor absorption from the stomach or intestines, deficient intake, and increased requirements. Decreased absorption may be due to pernicious anemia, surgical removal of the stomach, chronic inflammation of the pancreas, intestinal parasites, certain medications, and some genetic disorders. Medications that may decrease absorption include proton pump inhibitors and metformin. Decreased intake may occur in vegetarians or people who are malnourished. Increased requirements occur in people with HIV/AIDS, and in those with rapid red blood cell breakdown. Diagnosis is typically based on blood levels of vitamin B12. Elevated methylmalonic acid levels may also indicate a deficiency. A type of anemia known as megaloblastic anemia is often but not always present.
Treatment consists of using vitamin B12 by mouth or by injection; initially in high daily doses, followed by less frequent lower doses as the condition improves. If a reversible cause is found, that cause should be addressed if possible. If no reversible cause is found - or when found it cannot be eliminated - lifelong vitamin B12 administration is usually recommended. Vitamin B12 deficiency is preventable with supplements containing the vitamin: this is recommended in pregnant vegetarians and vegans, and not harmful in others. Risk of toxicity due to vitamin B12 is low.
Vitamin B12 deficiency in the US and the UK is estimated to occur in about 6 percent of those under the age of 60, and 20 percent of those over the age of 60. In Latin America, rates are estimated at 40 percent and they may be as high as 80 per cent in parts of Africa and Asia.
Signs and symptoms
Vitamin B12 deficiency can lead to anemia and neurological disorders.
A mild deficiency may not cause any discernible symptoms, but as the
deficiency becomes more significant, symptoms of anemia may result, such
as weakness, fatigue, light-headedness, rapid heartbeat, rapid
breathing and pale color to the skin. It may also cause easy bruising or bleeding, including bleeding gums, gastrointestinal
side effects including sore tongue, stomach upset, weight loss, and
diarrhea or constipation. If the deficiency is not corrected, nerve cell
damage can result. If this happens, vitamin B12 deficiency
may result in tingling or numbness to the fingers and toes, difficulty
walking, mood changes, depression, memory loss, disorientation and, in
severe cases, dementia.
The main type of vitamin B 12 deficiency anemia is pernicious anemia. It is characterized by a triad of symptoms:
- Anemia with bone marrow promegaloblastosis (megaloblastic anemia). This is due to the inhibition of DNA synthesis (specifically purines and thymidine)
- Gastrointestinal symptoms: alteration in bowel motility, such as mild diarrhea or constipation, and loss of bladder or bowel control. These are thought to be due to defective DNA synthesis inhibiting replication in a site with a high turnover of cells. This may also be due to the autoimmune attack on the parietal cells of the stomach in pernicious anemia. There is an association with GAVE syndrome (commonly called watermelon stomach) and pernicious anemia.
- Neurological symptoms: Sensory or motor deficiencies (absent reflexes, diminished vibration or soft touch sensation), subacute combined degeneration of spinal cord, seizures, or even symptoms of dementia and or other psychiatric symptoms may be present. Deficiency symptoms in children include developmental delay, regression, irritability, involuntary movements and hypotonia.
The presence of peripheral sensory-motor symptoms or subacute
combined degeneration of spinal cord strongly suggests the presence of a
B12 deficiency instead of folate deficiency. Methylmalonic acid, if not properly handled by B12,
remains in the myelin sheath, causing fragility. Dementia and
depression have been associated with this deficiency as well, possibly
from the under-production of methionine
because of the inability to convert homocysteine into this product.
Methionine is a necessary cofactor in the production of several neurotransmitters.
Each of those symptoms can occur either alone or along with others. The neurological complex, defined as myelosis funicularis, consists of the following symptoms:
- Impaired perception of deep touch, pressure and vibration, loss of sense of touch, very annoying and persistent paresthesias
- Ataxia of dorsal column type
- Decrease or loss of deep muscle-tendon reflexes
- Pathological reflexes — Babinski, Rossolimo and others, also severe paresis
Vitamin B12 deficiency can cause severe and irreversible
damage, especially to the brain and nervous system. These symptoms of
neuronal damage may not reverse after correction of blood abnormalities,
and the chance of complete reversal decreases with the length of time
the neurological symptoms have been present. Elderly people are at a even higher risk of this type of damage.
In babies a number of neurological symptoms can be evident due to
malnutrition or pernicious anemia in the mother. These include poor
growth, apathy, having no desire for food, and developmental regression.
While most symptoms resolve with supplementation some developmental and
cognitive problems may persist.
Only a small subset of dementia cases have been found to be reversible with vitamin B12 therapy. Tinnitus may be associated with vitamin B12 deficiency.
Causes
- Inadequate dietary intake of vitamin B12. Vitamin B12 occurs in animal products (eggs, meat, milk, fish) and in some edible algae. B12 isolated from bacterial cultures is also added to many fortified foods, and available as a dietary supplement. Vegans, and to a lesser degree vegetarians, may also be at risk for B12 deficiency due to inadequate dietary intake of B12, if they do not supplement. Children are at a higher risk for B12 deficiency due to inadequate dietary intake, as they have fewer vitamin stores and a relatively larger vitamin need per calorie of food intake.
- Selective impaired absorption of vitamin B12 due to intrinsic factor deficiency. This may be caused by the loss of gastric parietal cells in chronic atrophic gastritis (in which case, the resulting megaloblastic anemia takes the name of "pernicious anemia"), or may result from wide surgical resection of stomach (for any reason), or from rare hereditary causes of impaired synthesis of intrinsic factor. B12 deficiency is more common in the elderly because gastric intrinsic factor, necessary for absorption of the vitamin, is deficient, due to atrophic gastritis.
- Impaired absorption of vitamin B12 in the setting of a more generalized malabsorption or maldigestion syndrome. This includes any form due to structural damage or wide surgical resection of the terminal ileum (the principal site of vitamin B12 absorption).
- Forms of achlorhydria (including that artificially induced by drugs such as proton pump inhibitors and histamine 2 receptor antagonists) can cause B12 malabsorption from foods, since acid is needed to split B12 from food proteins and salivary binding proteins. This process is thought to be the most common cause of low B12 in the elderly, who often have some degree of achlorhydria without being formally low in intrinsic factor. This process does not affect absorption of small amounts of B12 in supplements such as multivitamins, since it is not bound to proteins, as is the B12 in foods.
- Surgical removal of the small bowel (for example in Crohn's disease) such that the patient presents with short bowel syndrome and is unable to absorb vitamin B12. This can be treated with regular injections of vitamin B12.
- Long-term use of ranitidine hydrochloride may contribute to deficiency of vitamin B12.
- Untreated celiac disease may also cause impaired absorption of this vitamin, probably due to damage to the small bowel mucosa. In some people, vitamin B12 deficiency may persist despite treatment with a gluten-free diet and require supplementation.
- Some bariatric surgical procedures, especially those that involve removal of part of the stomach, such as Roux-en-Y gastric bypass surgery. (Procedures such as the adjustable gastric band type do not appear to affect B12 metabolism significantly).
- Bacterial overgrowth within portions of the small intestine, such as may occur in blind loop syndrome, (a condition due to a loop forming in the intestine) may result in increased consumption of intestinal vitamin B12 by these bacteria.
- The diabetes medication metformin may interfere with B12 dietary absorption.
- A genetic disorder, transcobalamin II deficiency can be a cause.
- Alcoholism - if a "diet" of excessive alcohol intake is substituted for a diet adequate in sources of B12.
- Nitrous oxide exposure, and recreational use.
- Infection with the Diphyllobothrium latum tapeworm
- Chronic exposure to toxigenic molds and mycotoxins found in water damaged buildings.
- Increased needs by the body due to AIDS, or hemolysis the breakdown of red blood cells.
Mechanism
The total amount of vitamin B12 stored in the body is between two and five milligrams in adults. Approximately 50% is stored in the liver,
but approximately 0.1% is lost each day, due to secretions into the
gut—not all of the vitamin in the gut is reabsorbed. While bile is the main vehicle for B12 excretion, most of this is recycled via enterohepatic circulation. Due to the extreme efficiency of this mechanism, the liver can store three to five years worth of vitamin B12 under normal conditions and functioning. However, the rate at which B12 levels may change when dietary intake is low depends on the balance between several variables.
Metabolic
Vitamin B12 deficiency causes particular changes to the metabolism of two clinically relevant substances in humans:
- Homocysteine (homocysteine to methionine, catalysed by methionine synthase) leading to hyperhomocysteinemia
- Methylmalonic acid (methylmalonyl-CoA to succinyl-CoA, of which methylmalonyl-CoA is made from methylmalonic acid in a preceding reaction)
Methionine is activated to S-adenosyl methionine,
which aids in purine and thymidine synthesis, myelin production,
protein/neurotransmitters/fatty acid/phospholipid production and DNA
methylation. 5-Methyl tetrahydrofolate
provides a methyl group, which is released to the reaction with
homocysteine, resulting in methionine. This reaction requires cobalamin
as a cofactor. The creation of 5-methyl tetrahydrofolate is an
irreversible reaction. If B12 is absent, the forward reaction
of homocysteine to methionine does not occur, homocysteine
concentrations increase, and the replenishment of tetrahydrofolate
stops. Because B12 and folate are involved in the metabolism
of homocysteine, hyperhomocysteinuria is a non-specific marker of
deficiency. Methylmalonic acid is used as a more specific test of B12 deficiency.
Pathomorphology
A spongiform state of neural tissue along with edema of fibers and deficiency of tissue. The myelin decays, along with axial fiber. In later phases, fibric sclerosis
of nervous tissues occurs. Those changes apply to dorsal parts of the
spinal cord and to pyramidal tracts in lateral cords. The
pathophysiologic state of the spinal cord is called subacute combined degeneration of spinal cord.
In the brain itself, changes are less severe: They occur as small sources of nervous fibers decay and accumulation of astrocytes,
usually subcortically located, and also round hemorrhages with a torus
of glial cells. Pathological changes can be noticed as well in the
posterior roots of the cord and, to lesser extent, in peripheral nerves.
Abnormalities might be observed in MRI.
Diagnosis
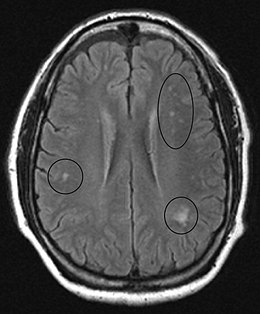
MRI image of the brain in vitamin B12 deficiency, axial view showing the "precontrast FLAIR image": note the abnormal lesions (circled) in the periventricular area suggesting white matter pathology.
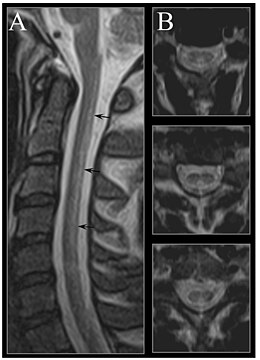
MRI image of the cervical spinal cord in vitamin B12
deficiency showing subacute combined degeneration. (A) The midsagittal
T2 weighted image shows linear hyperintensity in the posterior portion
of the cervical tract of the spinal cord (black arrows). (B) Axial T2
weighted images reveal the selective involvement of the posterior
columns.
Diagnosis is typically based on vitamin B12 blood levels below 120–180 picomol/L (170–250 pg/mL) in adults. Elevated methylmalonic acid levels (values >0.4 micromol/L) may also indicate a deficiency.
Serum homocysteine and methylmalonic acid levels are considered more reliable indicators of B12 deficiency than the concentration of B12 in blood. The levels of these substances are high in B12 deficiency and can be helpful if the diagnosis is unclear.
Routine monitoring of methylmalonic acid levels in urine is an option for people who may not be getting enough dietary B12, as a rise in methylmalonic acid levels may be an early indication of deficiency.
If nervous system damage is suspected, B12 analysis in cerebrospinal fluid is possible, though such an invasive test should be considered only if blood testing is inconclusive.
The Schilling test was a radio-isotope method, now outdated, of testing for low vitamin B12.
Effect of folic acid
The National Institutes of Health (NIH) has found that "Large amounts of folic acid can mask the damaging effects of vitamin B12 deficiency by correcting the megaloblastic anemia caused by vitamin B12
deficiency without correcting the neurological damage that also
occurs", there are also indications that "high serum folate levels might
not only mask vitamin B12 deficiency, but could also exacerbate the anemia and worsen the cognitive symptoms associated with vitamin B12 deficiency".
Due to the fact that in the United States legislation has required
enriched flour to contain folic acid to reduce cases of fetal
neural-tube defects, consumers may be ingesting more than they realize. To counter the masking effect of B12
deficiency the NIH recommends "folic acid intake from fortified food
and supplements should not exceed 1,000 μg daily in healthy adults." Most importantly, B12 deficiency needs to be treated with B12 repletion. Limiting folic acid will not counter the irrevocable neurological damage that is caused by untreated B12 deficiency.
Treatment
Hydroxocobalamin injection is a clear red liquid solution.
B12 can be supplemented by pill or injection and appears
to be equally effective in those with low levels due to deficient
absorption of B12.
When large doses are given by mouth its absorption does not rely
on the presence of intrinsic factor or an intact ileum. Generally 1 to
2 mg daily is required as a large dose. Even pernicious anemia can be treated entirely by the oral route. These supplements carry such large doses of the vitamin that 1% to 5% of high oral doses of free crystalline B12 is absorbed along the entire intestine by passive diffusion.
Epidemiology
Vitamin B12
deficiency is quite common and widespread. In the US and UK, as around 6
percent of people have the deficiency, and this rises to around 20
percent in those over the age of sixty. In less developed countries the
rates are higher. For example across Latin America 40 percent, in some
parts of Africa 70 percent, and in some parts of India 70 and 80
percent.
History
Adapted from:
- 1849 - Thomas Addison first described a case of pernicious anemia.
- 1877 - William Osler and William Gardner first described a case of neuropathy in this condition.
- 1878 - Hayem first described large red cells in the peripheral blood in this condition, which he called "giant blood corpuscles", now called macrocytes.
- 1880 - Paul Ehrlich first identified megaloblasts in the bone marrow in this condition.
- 1887 - Ludwig Lichtheim first described a case of myopathy in this condition.
- 1926 - George Minot shared the 1934 Nobel Prize with William Murphy and George Whipple, for discovery of an effective treatment for pernicious anemia using liver concentrate, later found to contain a large amount of vitamin B12.
- 1929 - William Castle demonstrated that gastric juice contained an "intrinsic factor" which when combined with meat ingestion resulted in absorption of the vitamin in this condition.
Other animals
Ruminants, such as cows and sheep, absorb B12 synthesized by their gut bacteria. Sufficient amounts of cobalt need to be consumed for this B12 synthesis to occur.
In the early 20th century during the development for farming of the North Island Volcanic Plateau
of New Zealand, cattle suffered from what was termed "bush sickness".
It was discovered that the volcanic soils lacked the cobalt salts
essential for synthesis of the vitamin by their gut bacteria.
The "coast disease" of sheep in the coastal sand dunes of South Australia
in the 1930s was found to originate in nutritional deficiencies of the
trace elements, cobalt and copper. The cobalt deficiency was overcome by
the development of "cobalt bullets", dense pellets of cobalt oxide
mixed with clay given orally for lodging in the animal's rumen.