From Wikipedia, the free encyclopedia
The heart is a muscular organ in both humans and other animals, which pumps blood through the blood vessels of the circulatory system.[1] Blood provides the body with oxygen and nutrients, and also assists in the removal of metabolic wastes.[2] The heart is located in the middle compartment of the mediastinum in the chest.[3]
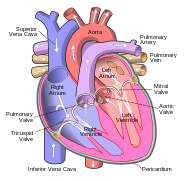
In humans, other mammals and birds the heart is divided into four chambers: upper left and right atria; and lower left and right ventricles.[4][5] Commonly the right atrium and ventricle are referred together as the right heart and their left counterparts as the left heart.[6] Fish in contrast have two chambers, an atrium and a ventricle, while reptiles have three chambers.[5] In a healthy heart blood flows one way through the heart due to heart valves, which prevent backflow.[3] The heart is enclosed in a protective sac, the pericardium, which also contains a small amount of fluid. The wall of the heart is made up of three layers: epicardium; myocardium; and endocardium.[7]
The heart pumps blood through both circulatory systems. Blood low in oxygen from the systemic circulation enters the right atrium from the superior and inferior vena cavae and passes to the right ventricle. From here it is pumped into the pulmonary circulation, through the lungs where it receives oxygen and gives off carbon dioxide. Oxygenated blood then returns to the left atrium, passes through the left ventricle and is pumped out through the aorta to the systemic circulation−where the oxygen is used and metabolized to carbon dioxide.[2] In addition the blood carries nutrients from the liver and gastrointestinal tract to various organs of the body, while transporting waste to the liver and kidneys.[citation needed] Normally with each heartbeat, the right ventricle pumps the same amount of blood into the lungs as the left ventricle pumps out into the body. Veins transport blood to the heart, while arteries transport blood away from the heart. Veins normally have lower pressures than arteries.[2][3] The heart contracts at a rate of around 72 beats per minute, at rest.[2] Exercise temporarily increases this rate, but lowers resting heart rate in the long term, and is good for heart health.[8]
Cardiovascular diseases (CVD) were the most common cause of death globally in 2008. CVD accounted for 30% of death cases during this year alone.[9][10] Of these deaths more than three quarters were due to coronary artery disease and stroke.[9] Risk factors include: smoking, being overweight, not enough exercise, high cholesterol, high blood pressure, and poorly controlled diabetes among others.[11] Diagnosis of CVD is often done by listening to the heart-sounds with a stethoscope, ECG or by ultrasound.[3] Diseases of the heart are primarily treated by cardiologists, although many specialties of medicine may be involved.[10]
Structure
Real-time MRI of the human heart
The heart is situated in the middle of the mediastinum behind the breastbone in the chest, at the level of thoracic vertebrae T5-T8. The largest part of the heart is usually slightly offset to the left (though occasionally it may be offset to the right). The heart is usually felt to be on the left side because the left heart is stronger, since it pumps to all body parts. The left lung in turn is smaller than the right lung because it has to accommodate the heart. The heart is supplied by the coronary circulation and is enclosed in the pericardial sac.
The pericardium encloses the heart and also attaches to the mediastinum via the pericardiac pleura, providing anchorage for the heart.[13] The back surface of the heart lies near to the vertebral column, and the front surface sits deep to the sternum and costal cartilages.[7] The two great veins, the venae cavae, and the great arteries, the aorta and pulmonary trunk, are attached to the upper surface of the heart, called the base, which is located at the level of the third costal cartilage.[7] The lower tip of the heart, the apex, lies just to the left of the sternum between the junction of the fourth and fifth ribs near their articulation with the costal cartilages.[7] The right side of the heart is deflected forwards, and the left side is deflected to the back.[7]
The shape of the heart is similar to a pinecone, rather broad at the base and tapering to the apex.[7] A stethoscope can be placed directly over the apex so that the beats can be counted. An adult heart has a mass of 250–350 grams (9–12 oz).[14] The heart is typically the size of a fist: 12 cm (5 in) in length, 8 cm (3.5 in) wide, and 6 cm (2.5 in) in thickness.[7] Well-trained athletes can have much larger hearts due to the effects of exercise on the heart muscle, similar to the response of skeletal muscle.[7]
Chambers
The heart has four chambers, two upper atria, the receiving chambers, and two lower ventricles, the discharging chambers. The atria are connected to the ventricles by the atrioventricular valves and they are separated by the coronary sulcus. The right atrium receives deoxygenated blood from the body and the left atrium receives oxygenated blood from the lungs. When these contract the blood is pushed into the ventricles, which pump to propel the blood to the lungs and the rest of the body.[7] There is an ear-shaped structure in the upper right atrium called the right atrial appendage, or auricle, and another in the upper left atrium, the left atrial appendage. The right atrium and the right ventricle together are sometimes referred to as the right heart and this sometimes includes the pulmonary trunk. Similarly, the left atrium and the left ventricle together are sometimes referred to as the left heart. These are separated by the posterior interventricular sulcus. The left heart pumps to the systemic circulation and the right heart pumps to the pulmonary circulation.
The cardiac skeleton is made of dense connective tissue and this gives structure to the heart and forms the atrioventricular septum which separates the right from the left heart, and the fibrous rings which serve as bases for the four heart valves.[15] The cardiac skeleton also provides an important boundary in the heart’s electrical conduction system since collagen cannot conduct electricity. The chordae tendinae attach to the atrioventricular valve cusps. The interatrial septum separates the atria and the interventricular septum separates the ventricles.[7] These septa (dividing walls) develop from the myocardium to form the heart chambers. The interventricular septum is much thicker than the interatrial septum, since the ventricles need to generate greater pressure when they contract.[7]
Valves
With the atria and major vessels removed, all four valves are clearly visible, although it is difficult to distinguish the three separate cusps of the tricuspid valve.[7]
Schematic of the heart showing valves, arteries and veins. The white arrows shows the normal direction of blood flow.

Frontal section showing papillary muscles attached to the tricuspid valve on the right and to the mitral valve on the left via chordae tendineae.[7]
All four heart valves lie along the same plane. The valves ensure unidirectional blood flow through the heart and prevent backflow. Between the right atrium and the right ventricle is the tricuspid valve. The right ventricle receives blood from the right atrium through the tricuspid valve. This consists of three cusps (flaps or leaflets), made of endocardium reinforced with additional connective tissue. Each of the three valve-cusps is attached to several strands of connective tissue, the chordae tendineae (tendinous cords), sometimes referred to as the heart strings. They are composed of approximately 80 percent collagenous fibers with the remainder consisting of elastic fibers and endothelium. They connect each of the cusps to a papillary muscle that extends from the lower ventricular surface. These muscles control the opening and closing of the valves. The three papillary muscles in the right ventricle are called the anterior, posterior, and septal muscles, which correspond to the three positions of the valve cusps.
Between the left atrium and left ventricle is the mitral valve, also known as the bicuspid valve due to its having two cusps, an anterior and a posterior medial cusp. These cusps are also attached via chordae tendinae to two papillary muscles projecting from the ventricular wall.
These two valves are the atrioventricular valves. During the relaxation phase of the cardiac cycle, the papillary muscles are also relaxed and the tension on the chordae tendineae is slight. However, as the ventricle contracts, so do the papillary muscles. This creates tension on the chordae tendineae, helping to hold the cusps of the atrioventricular valves in place and preventing them from being blown back into the atria.[7]
The semilunar pulmonary valve is located at the base of the pulmonary artery. This has three cusps which are not attached to any papillary muscles. When the ventricle relaxes blood flows back into the ventricle from the artery and this flow of blood fills the pocket-like valve, pressing against the cusps which close to seal the valve. The semilunar aortic valve is at the base of the aorta and also is not attached to papillary muscles. This too has three cusps which close with the pressure of the blood flowing back from the aorta.[7]
Right heart
The two major systemic veins, the superior and inferior venae cavae, and the collection of veins that make up the coronary sinus which drains the myocardium, empty into the right atrium. The superior vena cava drains blood from above the diaphragm and empties into the upper back part of the right atrium. The inferior cava drains the blood from below the diaphragm and empties into the back part of the atrium below the opening for the superior cava. Immediately above and to the middle of the opening of the inferior cava is the opening of the thin-walled coronary sinus.[7]In the wall of the right atrium is an oval-shaped depression known as the fossa ovalis, which is a remnant of an opening in the fetal heart known as the foramen ovale. The foramen ovale allowed blood in the fetal heart to pass directly from the right atrium to the left atrium, allowing some blood to bypass the pulmonary circuit. Within seconds after birth, a flap of tissue known as the septum primum that previously acted as a valve closes the foramen ovale and establishes the typical cardiac circulation pattern.[7] Most of the internal surface of the right atrium is smooth, the depression of the fossa ovalis is medial, and the anterior surface has prominent ridges of pectinate muscles, which are also present in the right atrial appendage.[7]
The atria receive venous blood on a nearly continuous basis, preventing venous flow from stopping while the ventricles are contracting. While most ventricular filling occurs while the atria are relaxed, they do demonstrate a contractile phase when they actively pump blood into the ventricles just prior to ventricular contraction. The right atrium is connected to the right ventricle by the tricuspid valve.[7]
When the myocardium of the ventricle contracts, pressure within the ventricular chamber rises. Blood, like any fluid, flows from higher pressure to lower pressure areas, in this case, toward the pulmonary trunk and the atrium. To prevent any potential backflow, the papillary muscles also contract, generating tension on the chordae tendineae. This prevents the flaps of the valves from being forced into the atria and regurgitation of the blood back into the atria during ventricular contraction.[7]
The walls of the right ventricle are lined with trabeculae carneae, ridges of cardiac muscle covered by endocardium. In addition to these muscular ridges, a band of cardiac muscle, also covered by endocardium, known as the moderator band reinforces the thin walls of the right ventricle and plays a crucial role in cardiac conduction. It arises from the lower part of the interventricular septum and crosses the interior space of the right ventricle to connect with the inferior papillary muscle.[7]
When the right ventricle contracts, it ejects blood into the pulmonary trunk, which branches into the left and right pulmonary arteries that carry it to each lung. The upper surface of the right ventricle begins to taper as it approaches the pulmonary trunk. At the base of the pulmonary trunk is the pulmonary semilunar valve that prevents backflow from the pulmonary trunk.[7]
Left heart
After gas exchange in the pulmonary capillaries, blood returns to the left atrium high in oxygen via one of the four pulmonary veins. Only the left atrial appendage contains pectinate muscles. Blood flows nearly continuously from the pulmonary veins back into the atrium, which acts as the receiving chamber, and from here through an opening into the left ventricle. Most blood flows passively into the heart while both the atria and ventricles are relaxed, but toward the end of the ventricular relaxation period, the left atrium will contract, pumping blood into the ventricle. This atrial contraction accounts for approximately 20 percent of ventricular filling. The left atrium is connected to the left ventricle by the mitral valve.[7]Although both sides of the heart will pump the same amount of blood, the muscular layer is much thicker in the left ventricle compared to the right, due to the greater force needed here. Like the right ventricle, the left also has trabeculae carneae, but there is no moderator band. The left ventricle is the major pumping chamber for the systemic circuit; it ejects blood into the aorta through the aortic semilunar valve.[7]
Membranes, surface features, and layers
Membranes
The double membrane that surrounds the heart and roots of the great vessels is called the pericardium or pericardial sac.[7] The pericardium, (which means around the heart), consists of two layers: the outer fibrous pericardium and the inner serous pericardium[16] and these enclose the pericardial cavity. The fibrous pericardium is made of tough, dense connective tissue that protects the heart and maintains its position in the thorax.[7] The more delicate visceral serous pericardium consists of two layers: the parietal pericardium, which is fused to the fibrous pericardium, and an inner visceral pericardium, or epicardium, which is fused to the heart and is part of the heart wall. The pericardial cavity, filled with lubricating serous fluid, lies between the epicardium and the pericardium.[7]
In most internal organs, visceral serous membranes are microscopic.[7] However, in the heart, the epicardium is macroscopic. It consists of a simple squamous epithelium called mesothelium, reinforced with loose, irregular, or areolar connective tissue that attaches to the pericardium. This mesothelium secretes the pericardial fluid which reduces friction as the heart contracts.[7] It also enables the heart to move in response to the movements of the diaphragm and lungs.[17]
Surface features
Inside the pericardium, the chambers and a series of sulci are visible. Major coronary blood vessels are located in these sulci. The deep coronary sulcus is located between the atria and ventricles.Between the left and right ventricles are two additional sulci that are not as deep as the coronary sulcus. On the front and back of the heart's surface are the anterior and posterior interventricular sulci.[7] These two grooves separate the ventricles.
Layers
The wall of the heart is composed of three layers.[7] From outer to inner, these are the epicardium, the myocardium, and the endocardium.[7]
The middle and thickest layer is the myocardium, made largely of cardiac muscle cells. It is built upon a framework of collagenous fibers, plus the blood vessels that supply the myocardium and the nerve fibers that help regulate the heart.[7] It is the contraction of the myocardium that pumps blood through the heart and into the major arteries.[7] The muscle pattern is elegant and complex, as the muscle cells swirl and spiral around the chambers of the heart.[7] They form a figure 8 pattern around the atria and around the bases of the great vessels.[7] Deeper ventricular muscles also form a figure 8 around the two ventricles and proceed toward the apex. More superficial layers of ventricular muscle wrap around both ventricles.[7] This complex swirling pattern allows the heart to pump blood more effectively than a simple linear pattern would.[7]
The innermost layer of the heart wall, the endocardium, is joined to the myocardium with a thin layer of connective tissue. The endocardium lines the heart chambers and valves.[7] It is made of simple squamous epithelium called endothelium, which is continuous with the endothelial lining of the blood vessels.[7]
Once regarded simply as a lining, evidence indicates that the endothelium may play an active role in regulating the contraction of the myocardium.[7] The endothelium may also regulate the ongoing growth patterns of the cardiac muscle cells. The endothelins it secretes, create an environment in the surrounding tissue fluids that regulates ionic concentrations and states of contractility.[7] Endothelins are potent vasoconstrictors and establish a homeostatic balance with other vasoconstrictors and vasodilators.[7]
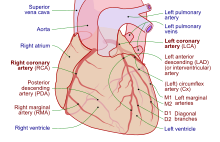
Coronary circulation
Cardiomyocytes like all other cells need to be supplied with oxygen, nutrients and a way of removing metabolic wastes. This is achieved by the coronary circulation. The coronary circulation cycles in peaks and troughs relating to the heart muscle relaxing or contracting.[7]Coronary arteries supply blood to the heart and the coronary veins remove the deoxygenated blood. There is a left and a right coronary artery supplying the left and right hearts respectively, and the septa. Smaller branches of these arteries anastomose, which in other parts of the body serve to divert blood due to a blockage. In the heart these are very small and cannot form other interconnections with the result that a coronary artery blockage can cause a myocardial infarction and with it, tissue damage.[7]
The great cardiac vein receives the major branches of the posterior, middle, and small cardiac veins and drains into the coronary sinus a large vein that empties into the right atrium. The anterior cardiac veins drain the front of the right ventricle and drain directly into the right atrium.[7]
Development
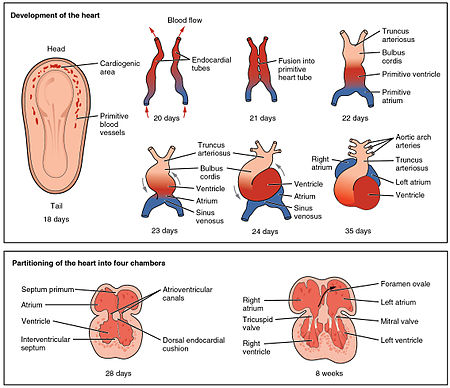
An outline of the embryological development of the human heart during the first eight weeks and the subsequent formation of the four heart chambers.[7]
The heart is the first functional organ to develop and starts to beat and pump blood at about three weeks into embryogenesis. This early start is crucial for subsequent embryonic and prenatal development.
The heart derives from splanchnopleuric mesenchyme in the neural plate which forms the cardiogenic region. Two endocardial tubes form here that fuse to form a primitive heart tube known as the tubular heart.[18] This quickly differentiates into five regions, from head to tail - the truncus arteriosus, bulbus cordis, primitive ventricle, primitive atrium, and the sinus venosus.[7] The truncus arteriosus will divide to form the aorta and pulmonary artery; the bulbus cordis will develop into the right ventricle; the primitive ventricle will form the left ventricle; the primitive atrium will become the front parts of the left and right atria and their appendages, and the sinus venosus will develop into the posterior part of the right atrium, the sinoatrial node and the coronary sinus.[7]
Initially, all venous blood flows into the sinus venosus, and contractions propel the blood from the sinus venosus to the truncus arteriosus.[7] Between the third and fourth week, the heart tube lengthens, and begins to fold to form an S-shape within the pericardium. This places the chambers and major vessels into the correct alignment for the developed heart. Further development will include the septa and valves formation and remodelling of the heart chambers. By the end of the fifth week the septa are complete and the heart valves are completed by the ninth week.[7]
The embryonic heart begins beating at around 22 days after conception (5 weeks after the last normal menstrual period, LMP). It starts to beat at a rate near to the mother’s which is about 75–80 beats per minute (bpm). The embryonic heart rate then accelerates linearly by approximately 3.3 bpm every day, or about 10 BPM every three days, which gives an increase of 100 bpm during the first month which peaks at 165–185 bpm early in the early 7th week (early 9th week after the LMP). The regression formula which describes this acceleration before the embryo reaches 25 mm in crown-rump length or 9.2 LMP weeks is: Age in days = EHR(0.3)+6 [19][20][21]
After 9 weeks (start of the fetal stage) it starts to decelerate, slowing to around 145 (±25) bpm at birth. There is no difference in female and male heart rates before birth.[22]
Physiology
 Play media
Play media
Blood flow through the heart from the Khan academy
The function of the heart involves blood flow; myocardium structure; the electrical conduction system of the heart; the cardiac cycle and cardiac output.
Blood flow
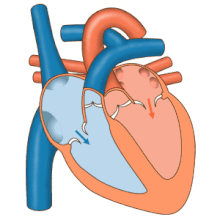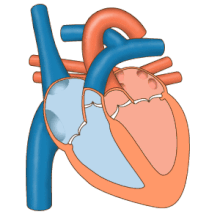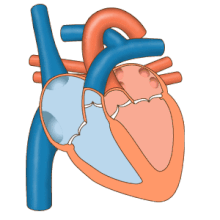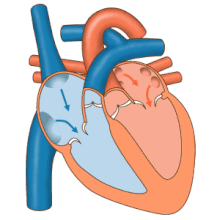

3D echocardiogram viewed from the top, with the upper part of the ventricles removed and the mitral valve clearly visible (cusps are not clear and pulmonary valve not visible). On the left are two, two-dimensional views showing tricuspid and mitral valves (above) and aortic valve (below).
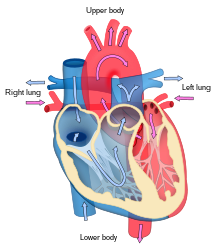
The heart functions as a pump and acts as a double pump in the cardiovascular system to provide a continuous circulation of blood throughout the body. This circulation includes the systemic circulation and the pulmonary circulation. Both circuits transport blood but they can also be seen in terms of the gases they carry. The pulmonary circulation collects oxygen from the lungs and delivers carbon dioxide for exhalation.The systemic circuit transports oxygen to the body and returns relatively deoxygenated blood and carbon dioxide to the pulmonary circuit.[7]
Blood flows through the heart in one direction, from the atria to the ventricles, and out through the pulmonary artery into the pulmonary circulation, and the aorta into the systemic circulation. The pulmonary artery (also trunk) branches into the left and right pulmonary arteries to supply each lung. Blood is prevented from flowing backwards (regurgitation) by the tricuspid, bicuspid, aortic, and pulmonary valves.
The function of the right heart, is to collect de-oxygenated blood, in the right atrium, from the body (via the superior and inferior venae cavae and pump it, through the tricuspid valve, via the right ventricle, through the semilunar pulmonary valve and into the pulmonary artery in the pulmonary circulation where carbon dioxide can be exchanged for oxygen in the lungs.This happens through the passive process of diffusion. In the left heart oxygenated blood is returned to the left atrium via the pulmonary vein. It is then pumped into the left ventricle through the bicuspid valve and into the aorta for systemic circulation. Eventually in the systemic capillaries exchange with the tissue fluid and cells of the body occurs; oxygen and nutrients are supplied to the cells for their metabolism and exchanged for carbon dioxide and waste products[7] In this case, oxygen and nutrients exit the systemic capillaries to be used by the cells in their metabolic processes, and carbon dioxide and waste products will enter the blood.[7]
The ventricles are stronger and thicker than the atria, and the muscle wall surrounding the left ventricle is thicker than the wall surrounding the right ventricle due to the higher force needed to pump the blood through the systemic circulation. Atria facilitate circulation primarily by allowing uninterrupted venous flow to the heart, preventing the inertia of interrupted venous flow that would otherwise occur at each ventricular systole.[23]
Cardiac muscle
Cardiac muscle tissue has autorhythmicity, the unique ability to initiate a cardiac action potential at a fixed rate - spreading the impulse rapidly from cell to cell to trigger the contraction of the entire heart. This autorhythmicity is still modulated by the endocrine and nervous systems.[7]There are two types of cardiac muscle cell: cardiomyocytes which have the ability to contract easily, and modified cardiomyocytes the pacemaker cells of the conducting system. The cardiomyocytes make up the bulk (99%) of cells in the atria and ventricles. These contractile cells respond to impulses of action potential from the pacemaker cells and are responsible for the contractions that pump blood through the body. The pacemaker cells make up just (1% of cells) and form the conduction system of the heart. They are generally much smaller than the contractile cells and have few of the myofibrils or myofilaments which means that have limited contractibility. Their function is similar in many respects to neurons.[7] The bundle of His and Purkinje fibres are specialised cardiomyocytes that function in the conduction system.
Electrical conduction

Transmission of a cardiac action potential through the heart's conduction system
It is not very well known how the electric signal moves in the atria. It seems that it moves in a radial way, but Bachmann's bundle and coronary sinus muscle play a role in conduction between the two atria, which have a nearly simultaneous systole.[24][25][26] While in the ventricles, the signal is carried by specialized tissue called the Purkinje fibers which then transmit the electric charge to the myocardium.[27]
If embryonic heart cells are separated into a Petri dish and kept alive, each is capable of generating its own electrical impulse followed by contraction. When two independently beating embryonic cardiac muscle cells are placed together, the cell with the higher inherent rate sets the pace, and the impulse spreads from the faster to the slower cell to trigger a contraction. As more cells are joined together, the fastest cell continues to assume control of the rate. A fully developed adult heart maintains the capability of generating its own electrical impulse, triggered by the fastest cells, as part of the cardiac conduction system. The components of the cardiac conduction system include the atrial and ventricular syncytium, the sinoatrial node, the atrioventricular node, the bundle of His (atrioventricular bundle), the bundle branches, and the Purkinje cells.[7]
Sinoatrial (SA) node

Normal sinus rhythm is established by the sinoatrial (SA) node, the heart's pacemaker. The SA node is a specialized grouping of cardiomyocytes in the upper and back walls of the right atrium very close to the opening of the superior vena cava. The SA node has the highest rate of depolarization.[7]
The atrioventricular (AV) node is a second cluster of specialized myocardial conductive cells, located in the inferior portion of the right atrium within the atrioventricular septum. The septum prevents the impulse from spreading directly to the ventricles without passing through the AV node. There is a critical pause before the AV node depolarizes and transmits the impulse to the atrioventricular bundle. This delay in transmission is partially attributable to the small diameter of the cells of the node, which slow the impulse. Also, conduction between nodal cells is less efficient than between conducting cells.[7]
Membrane potentials and ion movement in cardiac conductive cells
Action potentials are considerably different between conductive and contractive cardiomyocytes.While sodium Na+ and potassium K+ ions play essential roles, calcium ions Ca2+ are also critical for both types of cell. Unlike skeletal muscles and neurons, cardiac conductive cells do not have a stable resting potential. Conductive cells contain a series of sodium ion channels that allow a normal and slow influx of sodium ions that causes the membrane potential to rise slowly from an initial value of −60 mV up to about –40 mV. The resulting movement of sodium ions creates spontaneous depolarization (or prepotential depolarization).[7]
At this point, calcium channels open and Ca2+ enters the cell, further depolarizing it at a more rapid rate until it reaches a value of approximately +5 mV. At this point, the calcium ion channels close and potassium channels open, allowing outflux of K+ and resulting in repolarization. When the membrane potential reaches approximately −60 mV, the K+ channels close and Na+ channels open, and the prepotential phase begins again. This process gives the autorhythmicity to cardiac muscle.[7]

The prepotential is due to a slow influx of sodium ions until the threshold is reached followed by a rapid depolarization and repolarization. The prepotential accounts for the membrane reaching threshold and initiates the spontaneous depolarization and contraction of the cell; there is no resting potential.[7]
Calcium ions
Calcium ions play two critical roles in the physiology of cardiac muscle. Their influx through slow calcium channels accounts for the prolonged plateau phase and absolute refractory period. Calcium ions also combine with the regulatory protein troponin C in the troponin complex to enable contraction of the cardiac muscle, and separate from the protein to allow relaxation.[28] Different troponin types interact with each other, acting as intermediaries between calcium ions and the filament protein tropomyosin. When calcium ions are present, they unite to troponin C provoking changes in the complex that lead to tropomyosine uncovering sites in the filament where myosin molecules bind to start the contraction.[29] Both roles enable the myocardium to function properly.[7]Approximately 20 percent of the calcium required for contraction is supplied by the influx of Ca2+ during the plateau phase. The remaining Ca2+ for contraction is released from storage in the sarcoplasmic reticulum.[7]
Comparative rates of conduction system firing
The pattern of prepotential or spontaneous depolarization, followed by rapid depolarization and repolarization just described, are seen in the SA node and a few other conductive cells in the heart. Since the SA node is the pacemaker, it reaches threshold faster than any other component of the conduction system. It will initiate the impulses spreading to the other conducting cells. The SA node, without nervous or endocrine control, would initiate a heart impulse approximately 80–100 times per minute. Although each component of the conduction system is capable of generating its own impulse, the rate progressively slows from the SA node to the Purkinje fibers. Without the SA node, the AV node would generate a heart rate of 40–60 beats per minute. If the AV node were blocked, the atrioventricular bundle would fire at a rate of approximately 30–40 impulses per minute. The bundle branches would have an inherent rate of 20–30 impulses per minute, and the Purkinje fibers would fire at 15–20 impulses per minute. While a few exceptionally trained aerobic athletes demonstrate resting heart rates in the range of 30–40 beats per minute (the lowest recorded figure is 28 beats per minute for Miguel Indurain, a cyclist)–for most individuals, rates lower than 50 beats per minute would indicate a condition called bradycardia. Depending upon the specific individual, as rates fall much below this level, the heart would be unable to maintain adequate flow of blood to vital tissues, initially resulting in decreasing loss of function across the systems, unconsciousness, and ultimately death.[7]Cardiac cycle

The period of time that begins with contraction of the atria and ends with ventricular relaxation is known as the cardiac cycle. The period of contraction that the heart undergoes while it pumps blood into circulation is called systole. The period of relaxation that occurs as the chambers fill with blood is called diastole. Both the atria and ventricles undergo systole and diastole, and it is essential that these components be carefully regulated and coordinated to ensure blood is pumped efficiently to the body.[7]
Pressures and flow
Fluids, move from regions of high pressure to regions of lower pressure. Accordingly, when the heart chambers are relaxed (diastole), blood will flow into the atria from the higher pressure of the veins.As blood flows into the atria, the pressure will rise, so the blood will initially move passively from the atria into the ventricles. When the action potential triggers the muscles in the atria to contract (atrial systole), the pressure within the atria rises further, pumping blood into the ventricles. During ventricular systole, pressure rises in the ventricles, pumping blood into the pulmonary trunk from the right ventricle and into the aorta from the left ventricle.[7]
Phases of the cardiac cycle
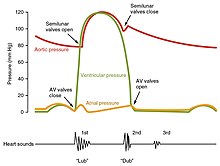
At the beginning of the cardiac cycle, both the atria and ventricles are relaxed (diastole). Blood is flowing into the right atrium from the superior and inferior venae cavae and the coronary sinus. Blood flows into the left atrium from the four pulmonary veins. The two atrioventricular valves, the tricuspid and mitral valves, are both open, so blood flows unimpeded from the atria and into the ventricles. Approximately 70–80 percent of ventricular filling occurs by this method. The two semilunar valves, the pulmonary and aortic valves, are closed, preventing backflow of blood into the right and left ventricles from the pulmonary trunk on the right and the aorta on the left.[7]Atrial systole and diastole
Contraction of the atria follows depolarization, represented by the P wave of the ECG. As the atrial muscles contract from the superior portion of the atria toward the atrioventricular septum, pressure rises within the atria and blood is pumped into the ventricles through the open atrioventricular (tricuspid, and mitral or bicuspid) valves. At the start of atrial systole, the ventricles are normally filled with approximately 70–80 percent of their capacity due to inflow during diastole. Atrial contraction, also referred to as the "atrial kick," contributes the remaining 20–30 percent of filling. Atrial systole lasts approximately 100 ms and ends prior to ventricular systole, as the atrial muscle returns to diastole.[7]Heart sounds
One of the simplest methods of assessing the heart's condition is to listen to it using a stethoscope.[7] In a healthy heart, there are only two audible heart sounds, called S1 and S2. The first heart sound S1, is the sound created by the closing of the atrioventricular valves during ventricular contraction and is normally described as "lub". The second heart sound, S2, is the sound of the semilunar valves closing during ventricular diastole and is described as "dub".[7] Each sound consists of two components, reflecting the slight difference in time as the two valves close.[30] S2 may split into two distinct sounds, either as a result of inspiration or different valvular or cardiac problems.[30] Additional heart sounds may also be present and these give rise to gallop rhythms. A third heart sound, S3 usually indicates an increase in ventricular blood volume. A fourth heart sound S4 is referred to as an atrial gallop and is produced by the sound of blood being forced into a stiff ventricle. The combined presence of S3 and S4 give a quadruple gallop.[7]Heart murmurs are abnormal heart sounds which can be either pathological or benign and there are numerous kinds.[31] Murmurs are graded by volume, from 1) the quietest, to 6) the loudest, and evaluated by their relationship to the heart sounds and position in the cardiac cycle.[30] Phonocardiograms can record these sounds.[7] Murmurs can result from narrowing (stenosis), regurgitation or insufficiency of any of the main heart valves but they can also result from a number of other disorders, including atrial and ventricular septal defects.[30] One example of a murmur is Still's murmur, which presents a musical sound in children, has no symptoms and disappears in adolescence.[32]
A different type of sound, a pericardial friction rub can be heard in cases of pericarditis where the inflamed membranes can rub together.[33]
Heart rate
The resting heart rate of a newborn can be 120 beats per minute (bpm) and this gradually decreases until maturity and then gradually increases again with age. The adult resting heart rate ranges from 60-100 bpm. Exercise and fitness levels, age and basal metabolic rate can all affect the heart rate. An athlete’s heart rate can be lower than 60bpm. During exercise the rate can be 150bpm with maximum rates reaching from 200 and 220 bpm.[7]Cardiovascular centres
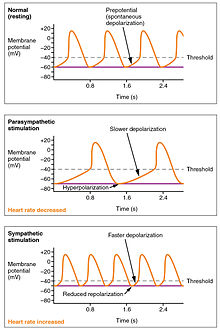
The wave of depolarization in a normal sinus rhythm shows a stable resting HR. Following parasympathetic stimulation, HR slows. Following sympathetic stimulation, HR increases.[7]
The normal sinus rhythm of the heart rate is generated by the SA node. It is also influenced by central factors through sympathetic and parasympathetic nerves[34] of the two paired cardiovascular centres of the medulla oblongata. Activity is increased via sympathetic stimulation of the cardioaccelerator nerves, and inhibited via parasympathetic stimulation by the vagus nerve. During rest vagal stimulation normally predominates as, left unregulated, the SA node would initiate a sinus rhythm of approximately 100 bpm.[7]
Both sympathetic and parasympathetic stimuli flow through the paired cardiac plexus near the base of the heart. Without any nervous stimulation, the SA node would establish a sinus rhythm of approximately 100 bpm. Since resting rates are considerably less than this, it becomes evident that parasympathetic stimulation normally slows HR.[7] The cardioaccelerator center also sends additional fibers, forming the cardiac nerves via sympathetic ganglia (the cervical ganglia plus superior thoracic ganglia T1–T4) to both the SA and AV nodes, plus additional fibers to the atria and ventricles. The ventricles are more richly innervated by sympathetic fibers than parasympathetic fibers. Sympathetic stimulation causes the release of the neurotransmitter norepinephrine (also known as noradrenaline) at the neuromuscular junction of the cardiac nerves. This shortens the repolarization period, thus speeding the rate of depolarization and contraction, which results in an increased heartrate. It opens chemical or ligand-gated sodium and calcium ion channels, allowing an influx of positively charged ions.[7] Norepinephrine binds to the beta–1 receptor. High blood pressure medications are used to block these receptors and so reduce the heart rate.[7]
The cardiovascular centres receive input from a series of visceral receptors with impulses traveling through visceral sensory fibers within the vagus and sympathetic nerves via the cardiac plexus. Among these receptors are various proprioreceptors, baroreceptors, and chemoreceptors, plus stimuli from the limbic system which normally enable the precise regulation of heart function, via cardiac reflexes. Increased physical activity results in increased rates of firing by various proprioreceptors located in muscles, joint capsules, and tendons. The cardiovascular centres monitor these increased rates of firing, suppressing parasympathetic stimulation or increasing sympathetic stimulation as needed in order to increase blood flow.[7]
Similarly, baroreceptors are stretch receptors located in the aortic sinus, carotid bodies, the venae cavae, and other locations, including pulmonary vessels and the right side of the heart itself. Rates of firing from the baroreceptors represent blood pressure, level of physical activity, and the relative distribution of blood. The cardiac centers monitor baroreceptor firing to maintain cardiac homeostasis, a mechanism called the baroreceptor reflex. With increased pressure and stretch, the rate of baroreceptor firing increases, and the cardiac centers decrease sympathetic stimulation and increase parasympathetic stimulation. As pressure and stretch decrease, the rate of baroreceptor firing decreases, and the cardiac centers increase sympathetic stimulation and decrease parasympathetic stimulation.[7]
There is a similar reflex, called the atrial reflex or Bainbridge reflex, associated with varying rates of blood flow to the atria. Increased venous return stretches the walls of the atria where specialized baroreceptors are located. However, as the atrial baroreceptors increase their rate of firing and as they stretch due to the increased blood pressure, the cardiac center responds by increasing sympathetic stimulation and inhibiting parasympathetic stimulation to increase HR. The opposite is also true.[7]
Factors influencing heart rate
In addition to the autonomic nervous system, other factors can impact on this. These include epinephrine, norepinephrine, and thyroid hormones; levels of various ions including calcium, potassium, and sodium; body temperature; hypoxia; and pH balance.[7]
|
||||||||||||||||||||||||||
|
Factors that increase heart rate also trigger an increase in stroke volume. As with skeletal muscles the heart can increase in size and efficiency with exercise.[7] Thus endurance athletes such as marathon runners may have a heart that has hypertrophied by up to 40%.[35] The difference between maximum and minimum cardiac outputs is known as the cardiac reserve and this measures the residual capacity to pump blood.[7] Heart rates may reach up to 185-195 in exercise, depending on how fit a person is.[35]
Cardiac output
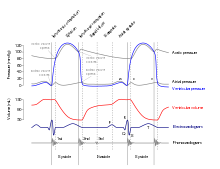
Cardiac output (CO) is a measurement of the amount of blood pumped by each ventricle (stroke volume, SV) in one minute. To calculate this, multiply stroke volume (SV), by heart rate (HR), in beats per minute.[7] It can be represented by the equation: CO = HR x SV[7]
SV is normally measured using an echocardiogram to record end diastolic volume (EDV) and end systolic volume (ESV), and calculating the difference: SV = EDV – ESV. SV can also be measured using a specialized catheter, but this is an invasive procedure and far more dangerous to the patient. A mean SV for a resting 70-kg (150-lb) individual would be approximately 70 mL. There are several important variables, including size of the heart, physical and mental condition of the individual, sex, contractility, duration of contraction, preload or EDV, and afterload or resistance. Normal range for SV would be 55–100 mL. An average resting HR would be approximately 75 bpm but could range from 60–100 in some individuals.[7] Using these numbers, (which refer to each ventricle, not both) the mean CO is 5.25 L/min, with a range of 4.0–8.0 L/min.[7]

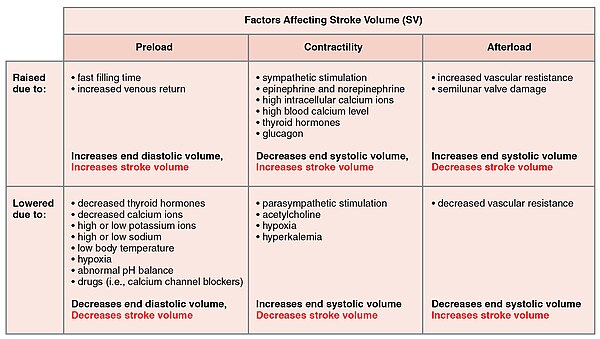
Major Factors Influencing Cardiac Output - Cardiac output is influenced by heart rate and stroke volume, both of which are also variable.[7]
SVs are also used to calculate ejection fraction, which is the portion of the blood that is pumped or ejected from the heart with each contraction. To calculate ejection fraction, SV is divided by EDV. Despite the name, the ejection fraction is normally expressed as a percentage. Ejection fractions range from approximately 55–70 percent, with a mean of 58 percent.[7]
Stroke volume
Major Factors Influencing Stroke Volume - Multiple factors impact preload, afterload, and contractility, and are the major considerations influencing SV.[7]
Summary of Major Factors Influencing Cardiac Output - The primary factors influencing HR include autonomic innervation plus endocrine control. Not shown are environmental factors, such as electrolytes, metabolic products, and temperature. The primary factors controlling SV include preload, contractility, and afterload. Other factors such as electrolytes may be classified as either positive or negative inotropic agents.[7]
Many of the factors that regulate the heart rate also have an impact on cardiac function by altering the stroke volume. While a number of variables are involved, stroke volume is dependent upon the difference between end diastolic volume and end systolic volume. The three primary factors involved are preload, afterload and contractility.[7]
Preload
Preload is another way of expressing EDV. Therefore, the greater the EDV, the greater the preload. A main factor is ventricular filling time. The faster the contractions are, the shorter the filling time and both the EDV and preload are lower.[7]The relationship between ventricular stretch and contraction has been stated in the Frank-Starling mechanism which says that the force of contraction is directly proportional to the initial length of muscle fibre. So that the greater the stretch of the ventricle the greater the contraction. Any sympathetic stimulation to the venous system will increase venous return to the heart and ventricular filling.[7]
Afterload
The ventricles must develop a certain tension to pump blood against the resistance of the vascular system. This tension is called afterload. When the resistance is increased particularly due to stenotic valve damage the afterload must necessarily increase. A decrease in normal vascular resistance can also occur. Different cardiac responses operate to restore homeostasis of the pressure and blood flow.[7]Contractility
The ability of the myocardium to contract, (its contractility), controls the stroke volume which determines the end systolic volume. The greater the contraction the greater the stroke volume and the smaller the end systolic volume. Positive or negative inotropic factors via sympathetic and parasympathetic stimulation respectively, can increase or decrease the force of contractions.Sympathetic stimulation triggers the release of norepinephrine from the cardiac nerves and also stimulates the adrenal cortex to secrete both epinephrine and norepinephrine. These secretions increase the heart rate, subsequent metabolic rate and contractility. Parasympathetic stimulation stimulates the release of acetylcholine (ACh) from the vagus nerve which decreases contractility, and stroke volume which increases end systolic volume.
Several synthetic drugs have been developed that can act either as a stimulant or inhibitor inotrope. The stimulant inotropes, such as Digoxin, cause higher concentrations of calcium ions which increase contractility. Excess calcium (hypercalcemia) is also a positive inotrope. Drugs that are negative inotropes include beta blockers and calcium channel blockers. Hypoxia, acidosis, hyperkalemia are also negative inotropic agents.
|
|||||||||||||||
|
Clinical significance
The stethoscope is used for auscultation of the heart, and is one of the most iconic symbols for medicine. Auscultation of heart sounds is done in order to diagnose a number of diseases - primarily by listening for heart murmurs.
Atherosclerosis is a condition affecting the cardiovascular system. If atherosclerosis occurs in the coronary arteries (which supply the heart) the result may be angina pectoris, or in worse cases a heart attack.
Auscultation of the chest area is a very useful diagnostic tool because of its noninvasiveness. Heart murmurs in this 3-year old girl revealed a tumorous mass in the right ventricle of the heart, in this case a cardiac rhabdomyoma.
Being such a complex organ the heart is prone to several cardiovascular diseases some becoming more prevalent with ageing.[36] Heart disease is a major cause of death, accounting for an average of 30% of all deaths in 2008, globally.[9] This rate varies from a lower 28% to a high 40% in high-income countries.[10]
Coronary artery disease is also known as ischemic heart disease (IHD), and more usually as atherosclerosis. This disease is caused by a build-up of plaque along the inner walls of the arteries which has the effect of narrowing the arteries and so reducing the blood flow to the heart. It is the most common form of heart disease, the cause of heart attacks and the most common cause of death, globally.[37] Coronary artery bypass surgery to improve the blood supply to the heart is often the only treatment option.
Cardiomyopathy is a noticeable deterioration of the heart muscle's ability to contract, which can lead to heart failure. The most common form of cardiomyopathy is dilated cardiomyopathy.[38][39]
Heart failure which can also be congestive heart failure, happens when the heart is pumping insufficiently and cannot meet the need of blood flow required by the body.[40] Because the heart is a double pump, each side can fail independently of the other, resulting in heart failure of the right heart or heart failure of the left heart either of which through causing strain in the other side can result in the failure of the whole heart. Congestive heart failure results in blood backing up in the systemic circulation. Edema (swelling) of the feet, ankles and fingers is the most noticeable symptom. Pulmonary congestion results from left heart failure. The right side of the heart continues to propel blood to the lungs, but the left side is unable to pump the returning blood into the systemic circulation. As blood vessels within the lungs become swollen with blood, the pressure within them increases, and fluid leaks from the circulation into the lung tissue, causing pulmonary edema. If untreated, the person will suffocate because they are drowning in their own blood.[41] Common causes of heart failure are a heart attack, valvular heart disease and hypertension.
Other conditions can interfere with the regular conduction of impulses across the heart. Damage to the sinoatrial node (SA), (the pace maker of the heart), can result in a slower heart rate. Ischemia, or an inadequate blood supply to the heart muscle, may lead to fibrillation - a rapid, uncoordinated shuddering of the heart muscle, a major cause of fatal heart attacks.[41]
Heart murmurs are abnormal or unusual heart sounds which can be caused by an obstruction in the blood flow. These murmurs can be heard with a stethoscope. Heart murmurs are common in young children and the elderly even if they have perfectly healthy hearts. They may have heart murmurs because their heart walls are thin and vibrate with the rushing blood. However, murmurs in patients who do not fall into either of those categories most often have a valve issue. For example, if a valve does not close tightly enough, a swishing sound will be heard after that valve has (supposedly) closed, as the blood flows back through the partially open valve. Distinct sounds also can be heard when blood flows turbulently through stenosed (narrowed) valves.[41]
Cardiac tamponade, also known as pericardial tamponade, is the condition of an abnormal build-up of fluid in the pericardium which can adversely affect the function of the heart. The fluid can be removed from the pericardial sac using a syringe in a procedure called pericardiocentesis.
Cardiac arrest is the sudden cessation of normal heart rhythm which can include a number of pathologies such as cardiac dysrhythmia, an irregular and ineffective heart rhythm which can be either an extremely rapid heart beat (tachycardia) or a very slow one (brachycardia), which prevents the heart from effectively pumping blood, and asystole, which is the cessation of heart rhythm entirely.
Exercise results in the addition of protein myofilaments and this can result in hypertrophy where the size of individual cells are increased but not their number.[7] This is a condition known as athletic heart syndrome. The hearts of athletes can pump more efficiently at lower heart rates. However, enlarged hearts can have a pathological cause which can result in a heart of 1000 g (2 lb) in mass. Hypertrophic cardiomyopathy is one such cause.[7] The cause of an abnormally enlarged heart muscle is unknown, but the condition is often undiagnosed and can cause sudden death in young athletes.[7]
Carditis is inflammation of the heart; this can be specific to regions as in pericarditis, myocarditis, and endocarditis or it can be of the whole heart known as pancarditis.
A rare type of cancer can form in the mesothelium of the pericardium known as a mesothelioma.[42]
Angiogenesis represents a therapeutic target for cardiovascular disease.[43]
Electrocardiogram

A normal ECG tracing.[7]
Using surface electrodes on the body, it is possible to record the complex electrical activity of the heart. This tracing of the electrical signal is the electrocardiogram (ECG) or (EKG). An ECG clearly shows normal and abnormal heart function and is an indispensable diagnostic tool.
There are five prominent points on the ECG: the P wave (atrial depolarisation), the QRS complex (atrial repolarisation and ventricular depolarisation) and the T wave (ventricular repolarisation).[7]
Lifestyle and heart health
Obesity, high blood pressure, and high cholesterol can all increase the risk of developing heart disease. However, half the number of heart attacks occur in people with normal cholesterol levels.It is generally accepted that factors such as exercise or the lack of it, good or poor diet, and overall well-being (including emotional), affect heart health.[44][45][46][47]
History
Ancient
The valves of the heart were discovered by a physician of the Hippocratean school around the 4th century BC, although their function was not fully understood. On dissection, arteries are typically empty of blood because blood pools in the veins after death. It was subsequently assumed they were filled with air and served to transport air around the body.
Philosophers distinguished veins from arteries, but thought the pulse was a property of arteries. Erasistratos observed that arteries cut during life bleed. He ascribed the fact to the phenomenon that air escaping from an artery is replaced with blood which entered by very small vessels between veins and arteries. Thus he apparently postulated capillaries, but with reversed flow of blood.
The Greek physician Galen (2nd century AD) knew blood vessels carried blood and identified venous (dark red) and arterial (brighter and thinner) blood, each with distinct and separate functions. Growth and energy were derived from venous blood created in the liver from chyle, while arterial blood gave vitality by containing pneuma (air) and originated in the heart. Blood flowed from both creating organs to all parts of the body, where it was consumed and there was no return of blood to the heart or liver. The heart did not pump blood around, the heart's motion sucked blood in during diastole and the blood moved by the pulsation of the arteries themselves.
Galen believed the arterial blood was created by venous blood passing from the left ventricle to the right through 'pores' in the interventricular septum, while air passed from the lungs via the pulmonary artery to the left side of the heart. As the arterial blood was created, "sooty" vapors were created and passed to the lungs, also via the pulmonary artery, to be exhaled.
Pre-modern
The earliest descriptions of the coronary and pulmonary circulation systems can be found in the Commentary on Anatomy in Avicenna's Canon, published in 1242 by Ibn al-Nafis.[48] In his manuscript, al-Nafis wrote that blood passes through the pulmonary circulation instead of moving from the right to the left ventricle as previously believed by Galen.[49] His work was later translated into Latin by Andrea Alpago.[50]In Europe, the teachings of Galen continued to dominate the academic community and his doctrines were adopted as the official canon of the Church. Andreas Vesalius questioned some of Galen's beliefs of the heart in De humani corporis fabrica (1543), but his magnum opus was interpreted as a challenge to the authorities and he was subjected to a number of attacks.[51] Michael Servetus wrote in Christianismi Restitutio (1553) that blood flows from one side of the heart to the other via the lungs, but his book was considered heresy and he was condemend and burned at the stake in Geneva.[51] The breakthrough came with the publication of De Motu Cordis (1628) by the English physician William Harvey. Harvey's book completely describes the systemic circulation and the mechanical force of the heart, leading to an overhaul of the Galenic doctrines.[52]
Modern
Otto Frank (1865–1944) was a German physiologist; among his many published works are detailed studies of this important heart relationship. Ernest Starling (1866–1927) was an important English physiologist who also studied the heart. Although they worked largely independently, their combined efforts and similar conclusions have been recognized in the name "Frank–Starling mechanism."[7]Although Purkinje fibres and the bundle of His were discovered as early as the 19th century, their specific role in the electrical conduction system of the heart remained unknown until Sunao Tawara published his monograph, titled Das Reizleitungssystem des Säugetierherzens, in 1906. Tawara's discovery of the atrioventricular node prompted Arthur Keith and Martin Flack to look for similar structures in the heart, leading to their discovery of the sinoatrial node several months later. These structures form the anatomical basis of the electrocardiogram, whose inventor, Willem Einthoven, was awarded the Nobel Prize in Medicine or Physiology in 1924.[53]
The first successful heart transplantation was performed in 1967 by the South African surgeon Christiaan Barnard at Groote Schuur Hospital in Cape Town. This marked an important milestone in cardiac surgery, capturing the attention of both the medical profession and the world at large. However, long-term survival rates of patients were initially very low. Louis Washkansky, the first recipient of a donated heart, died 18 days after the operation while other patients did not survive for more than a few weeks.[54] The American surgeon Norman Shumway has been credited for his efforts to improve transplantation techniques, along with pioneers Richard Lower, Vladimir Demikhov and Adrian Kantrowitz. As of March 2000, more than 55,000 heart transplantations have been performed worldwide.[55]
By the middle of the 20th century, heart disease had surpassed infectious disease as the leading cause of death in the United States, and it is currently the leading cause of deaths worldwide. Since 1948, the ongoing Framingham Heart Study has shed light on the effects of various influences on the heart, including diet, exercise, and common medications such as aspirin. Although the introduction of ACE inhibitors and beta blockers has improved the management of chronic heart failure, the disease continues to be an enormous medical and societal burden, with 30 to 40% of patients dying within a year of receiving the diagnosis.[56]
Society and culture
Symbolism
Letter ღ of the Georgian script is often used as a "heart" symbol.
The seal script glyph for "heart" (Middle Chinese sim)
As one of the vital organs, the heart was long identified as the center of the entire body, the seat of life, or emotion, or reason, will, intellect, purpose or the mind. Thus, in the Hebrew Bible, the word for "heart" לָבַב lebab is used in these meanings (paralleling the use of φρήν "diaphragm" in Homeric Greek).
An important part of the concept of the soul in Ancient Egyptian religion was thought to be the heart, or ib. The ib or metaphysical heart was believed to be formed from one drop of blood from the child's mother's heart, taken at conception.[57] To ancient Egyptians, the heart was the seat of emotion, thought, will, and intention. This is evidenced by Egyptian expressions which incorporate the word ib, such as Awt-ib for "happiness" (literally, "wideness of heart"), Xak-ib for "estranged" (literally, "truncated of heart").[citation needed] In Egyptian religion, the heart was the key to the afterlife. It was conceived as surviving death in the nether world, where it gave evidence for, or against, its possessor. It was thought that the heart was examined by Anubis and the deities during the Weighing of the Heart ceremony. If the heart weighed more than the feather of Maat, it was immediately consumed by the monster Ammit.
The Chinese character for "heart", 心, derives from a comparatively realistic depiction of a heart (indicating the heart chambers) in seal script. The Chinese word xīn also takes the metaphorical meanings of "mind, intelligence", "soul", or "center, core". In Chinese medicine, the heart is seen as the center of 神 shén "spirit, soul, consciousness".
The Sanskrit word for heart, hRd (हृद्), dates at least as far back as the Rigveda and is a cognate of the word for heart in Greek, Latin, and English. The same word is used to mean "mind" or "soul" depending on the context.
Many classical philosophers and scientists, including Aristotle, considered the heart the seat of thought, reason, or emotion, often disregarding the brain as contributing to those functions.[58] The identification of the heart as the seat of emotions in particular is due to the Roman physician Galen, who also located the seat of the passions in the liver, and the seat of reason in the brain.[59] However these "emotional properties" of the heart were later discovered to be solely centered in the brain. This tradition influenced the development of the medieval Christian devotion to the Sacred Heart of Jesus and the Immaculate Heart of Mary.
The idiomatic expression of "pierced" or "broken" hearts ultimately derive from devotional Christianity, where the hearts of Mary or Jesus are depicted as suffering various tortures (symbolizing the pain suffered by Christ for the sins of the world, and the pain of Mary at the crucifixion of her son, respectively), but from an early time the metaphor was transferred to unfulfilled romantic love, in late medieval literature dealing with the ideals of courtly love. The notion of "Cupid's arrows" is ancient, due to Ovid, but while Ovid describes Cupid as wounding his victims with his arrows, it is not made explicit that it is the heart that is wounded. The familiar iconography of Cupid shooting little heart symbols is Baroque.
Food
Animal hearts are widely consumed as food. As they are almost entirely muscle, they are high in protein. They are often included in dishes with other offal, for example in the pan-Ottoman kokoretsi.Chicken hearts are considered to be giblets, and are often grilled on skewers: Japanese hāto yakitori, Brazilian churrasco de curacao, Indonesian chicken heart satay.[60] They can also be pan-fried, as in Jerusalem mixed grill. In Egyptian cuisine, they can be used, finely chopped, as part of stuffing for chicken.[61] Many recipes combined them with other giblets, such as the Mexican pollo en menudencias[62] and the Russian ragu iz kurinyikh potrokhov.[63]
The hearts of beef, pork, and mutton can generally be interchanged in recipes. As heart is a hard-working muscle, it makes for "firm and rather dry" meat,[64] so is generally slow-cooked. Another way of dealing with toughness is to julienne the meat, as in Chinese stir-fried heart.[65]
Beef heart may be grilled or braised.[64] In the Peruvian anticuchos de corazón, barbecued beef hearts are grilled after being tenderized through long marination in a spice and vinegar mixture. An Australian recipe for "mock goose" is actually braised stuffed beef heart.[66]
Pig heart is stewed, poached, braised,[67] or made into sausage. The Balinese oret is a sort of blood sausage made with pig heart and blood. A French recipe for cœur de porc à l'orange is made of braised heart with an orange sauce.
Other animals
The structure of the heart can vary among the different animal species. Cephalopods have two "gill hearts" also known as branchial hearts and one "systemic heart". The vertebrate heart lies in the front (ventral) part of the body cavity, dorsal to the gut. It is always surrounded by a pericardium, which is usually a distinct structure, but may be continuous with the peritoneum in jawless and cartilaginous fish.The SA node is found in all amniotes but not in more primitive vertebrates. In these animals, the muscles of the heart are relatively continuous and the sinus venosus coordinates the beat which passes in a wave through the remaining chambers. Indeed, since the sinus venosus is incorporated into the right atrium in amniotes, it is likely homologous with the SA node. In teleosts, with their vestigial sinus venosus, the main centre of coordination is, instead, in the atrium. The rate of heartbeat varies enormously between different species, ranging from around 20 beats per minute in codfish to around 600 in hummingbirds[68] and up to 1200 bpm in the ruby-throated hummingbird.[69]
Double circulatory systems
In the heart of lungfish, the septum extends part-way into the ventricle. This allows for some degree of separation between the de-oxygenated bloodstream destined for the lungs and the oxygenated stream that is delivered to the rest of the body. The absence of such a division in living amphibian species may be partly due to the amount of respiration that occurs through the skin; thus, the blood returned to the heart through the vena cavae is already partially oxygenated. As a result, there may be less need for a finer division between the two bloodstreams than in lungfish or other tetrapods.Nonetheless, in at least some species of amphibian, the spongy nature of the ventricle does seem to maintain more of a separation between the bloodstreams. Also, the original valves of the conus arteriosus have been replaced by a spiral valve that divides it into two parallel parts, thereby helping to keep the two bloodstreams separate.[68]
Adult amphibians and most reptiles have a double circulatory system but the heart is not separated into two pumps. The development of the double system is necessitated by the presence of lungs which deliver oxygenated blood directly to the heart.
In amphibians, the atrium is divided into two chambers by a muscular septum but there is only one ventricle. The sinus venosus, which remains large, connects only to the right atrium and receives blood from the venae cavae, with the pulmonary vein by-passing it to enter the left atrium.
The heart of most reptiles is similar in structure to that of lungfish but the septum is generally much larger. This divides the ventricle into two halves but the septum does not reach the whole length of the heart and there is a considerable gap near the pulmonary artery and aorta openings. In most reptilian species, there appears to be little, if any, mixing between the bloodstreams, so the aorta receives, essentially, only oxygenated blood.[68]
The fully divided heart
Archosaurs (crocodilians and birds) and mammals show complete separation of the heart into two pumps for a total of four heart chambers; it is thought that the four-chambered heart of archosaurs evolved independently from that of mammals. In crocodilians, there is a small opening, the foramen of Panizza, at the base of the arterial trunks and there is some degree of mixing between the blood in each side of the heart, during a dive underwater;[70][71] thus, only in birds and mammals are the two streams of blood – those to the pulmonary and systemic circulations – permanently kept entirely separate by a physical barrier.[68]Fish

Primitive fish have a four-chambered heart, but the chambers are arranged sequentially so that this primitive heart is quite unlike the four-chambered hearts of mammals and birds. The first chamber is the sinus venosus, which collects deoxygenated blood, from the body, through the hepatic and cardinal veins. From here, blood flows into the atrium and then to the powerful muscular ventricle where the main pumping action will take place. The fourth and final chamber is the conus arteriosus which contains several valves and sends blood to the ventral aorta. The ventral aorta delivers blood to the gills where it is oxygenated and flows, through the dorsal aorta, into the rest of the body. (In tetrapods, the ventral aorta has divided in two; one half forms the ascending aorta, while the other forms the pulmonary artery).[68]
In the adult fish, the four chambers are not arranged in a straight row but, instead form an S-shape with the latter two chambers lying above the former two. This relatively simpler pattern is found in cartilaginous fish and in the ray-finned fish. In teleosts, the conus arteriosus is very small and can more accurately be described as part of the aorta rather than of the heart proper. The conus arteriosus is not present in any amniotes, presumably having been absorbed into the ventricles over the course of evolution. Similarly, while the sinus venosus is present as a vestigial structure in some reptiles and birds, it is otherwise absorbed into the right atrium and is no longer distinguishable.[68]
Invertebrates

The tube-like heart (green) of the mosquito Anopheles gambiae extends horizontally across the body, interlinked with the diamond-shaped wing muscles (also green) and surrounded by pericardial cells (red). Blue depicts cell nuclei.
Arthropods have an open circulatory system, and often some short open-ended arteries.The arthropod heart is typically a muscular tube that runs the length of the body, under the back and from the base of the head. Instead of blood the circulatory fluid is haemolymph which carries the most commonly used respiratory pigment, copper-based haemocyanin as the oxygen transporter; iron-based haemoglobin is used by only a few arthropods. The heart contracts in ripples from the rear to the front of the animal transporting water and nutrients. Pairs of valves run alongside the heart, allowing fluid to enter whilst preventing backflow.
In insects, the circulatory system is not used to transport oxygen and so is much reduced, having no veins or arteries and consisting of a single perforated tube running dorsally which pumps peristaltically. The simpler unsegmented invertebrates have no body cavity, and oxygen and nutrients pass through their bodies by diffusion.
Additional images
- Dissection