From Wikipedia, the free encyclopedia
| Stem cell | |
|---|---|
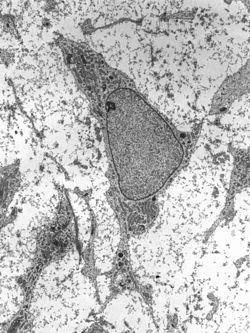
Transmission electron micrograph of an adult stem cell displaying typical ultrastructural characteristics.
|
|
| Details | |
| Latin | Cellula praecursoria |
| Identifiers | |
| Code | TH H2.00.01.0.00001 |
| TH | H1.00.01.0.00028, H2.00.01.0.00001 |
| FMA | 63368 |
| Anatomical terminology | |
Stem cells are undifferentiated biological cells that can differentiate into specialized cells and can divide (through mitosis) to produce more stem cells. They are found in multicellular organisms. In mammals, there are two broad types of stem cells: embryonic stem cells, which are isolated from the inner cell mass of blastocysts, and adult stem cells, which are found in various tissues. In adult organisms, stem cells and progenitor cells act as a repair system for the body, replenishing adult tissues. In a developing embryo, stem cells can differentiate into all the specialized cells—ectoderm, endoderm and mesoderm (see induced pluripotent stem cells)—but also maintain the normal turnover of regenerative organs, such as blood, skin, or intestinal tissues.
There are three known accessible sources of autologous adult stem cells in humans:
- Bone marrow, which requires extraction by harvesting, that is, drilling into bone (typically the femur or iliac crest).
- Adipose tissue (lipid cells), which requires extraction by liposuction.
- Blood, which requires extraction through apheresis, wherein blood is drawn from the donor (similar to a blood donation), and passed through a machine that extracts the stem cells and returns other portions of the blood to the donor.
Adult stem cells are frequently used in medical therapies, for example in bone marrow transplantation. Stem cells can now be artificially grown and transformed (differentiated) into specialized cell types with characteristics consistent with cells of various tissues such as muscles or nerves. Embryonic cell lines and autologous embryonic stem cells generated through Somatic-cell nuclear transfer or dedifferentiation have also been proposed as promising candidates for future therapies.[1] Research into stem cells grew out of findings by Ernest A. McCulloch and James E. Till at the University of Toronto in the 1960s.[2][3]
Properties
The classical definition of a stem cell requires that it possess two properties:- Self-renewal: the ability to go through numerous cycles of cell division while maintaining the undifferentiated state.
- Potency: the capacity to differentiate into specialized cell types. In the strictest sense, this requires stem cells to be either totipotent or pluripotent—to be able to give rise to any mature cell type, although multipotent or unipotent progenitor cells are sometimes referred to as stem cells. Apart from this it is said that stem cell function is regulated in a feed back mechanism.
Self-renewal
Two mechanisms exist to ensure that a stem cell population is maintained:- Obligatory asymmetric replication: a stem cell divides into one mother cell that is identical to the original stem cell, and another daughter cell that is differentiated.
- Stochastic differentiation: when one stem cell develops into two differentiated daughter cells, another stem cell undergoes mitosis and produces two stem cells identical to the original.
Potency definition

Pluripotent, embryonic stem cells originate as inner cell mass (ICM) cells within a blastocyst. These stem cells can become any tissue in the body, excluding a placenta. Only cells from an earlier stage of the embryo, known as the morula, are totipotent, able to become all tissues in the body and the extraembryonic placenta.
Potency specifies the differentiation potential (the potential to differentiate into different cell types) of the stem cell.[4]
- Totipotent (a.k.a. omnipotent) stem cells can differentiate into embryonic and extraembryonic cell types. Such cells can construct a complete, viable organism.[4] These cells are produced from the fusion of an egg and sperm cell. Cells produced by the first few divisions of the fertilized egg are also totipotent.[5]
- Pluripotent stem cells are the descendants of totipotent cells and can differentiate into nearly all cells,[4] i.e. cells derived from any of the three germ layers.[6]
- Multipotent stem cells can differentiate into a number of cell types, but only those of a closely related family of cells.[4]
- Oligopotent stem cells can differentiate into only a few cell types, such as lymphoid or myeloid stem cells.[4]
- Unipotent cells can produce only one cell type, their own,[4] but have the property of self-renewal, which distinguishes them from non-stem cells (e.g. progenitor cells, muscle stem cells).
Identification
In practice, stem cells are identified by whether they can regenerate tissue. For example, the defining test for bone marrow or hematopoietic stem cells (HSCs) is the ability to transplant the cells and save an individual without HSCs. This demonstrates that the cells can produce new blood cells over a long term. It should also be possible to isolate stem cells from the transplanted individual, which can themselves be transplanted into another individual without HSCs, demonstrating that the stem cell was able to self-renew.Properties of stem cells can be illustrated in vitro, using methods such as clonogenic assays, in which single cells are assessed for their ability to differentiate and self-renew.[7][8] Stem cells can also be isolated by their possession of a distinctive set of cell surface markers. However, in vitro culture conditions can alter the behavior of cells, making it unclear whether the cells will behave in a similar manner in vivo. There is considerable debate as to whether some proposed adult cell populations are truly stem cells.
Embryonic
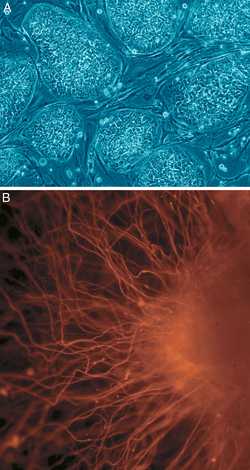
Embryonic stem (ES) cells are stem cells derived from the inner cell mass of a blastocyst, an early-stage embryo.[9] Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. ES cells are pluripotent and give rise during development to all derivatives of the three primary germ layers: ectoderm, endoderm and mesoderm. In other words, they can develop into each of the more than 200 cell types of the adult body when given sufficient and necessary stimulation for a specific cell type. They do not contribute to the extra-embryonic membranes or the placenta.Nearly all research to date has made use of mouse embryonic stem cells (mES) or human embryonic stem cells (hES). Both have the essential stem cell characteristics, yet they require very different environments in order to maintain an undifferentiated state. Mouse ES cells are grown on a layer of gelatin as an extracellular matrix (for support) and require the presence of leukemia inhibitory factor (LIF). Human ES cells are grown on a feeder layer of mouse embryonic fibroblasts (MEFs) and require the presence of basic fibroblast growth factor (bFGF or FGF-2).[10] Without optimal culture conditions or genetic manipulation,[11] embryonic stem cells will rapidly differentiate.
A human embryonic stem cell is also defined by the expression of several transcription factors and cell surface proteins. The transcription factors Oct-4, Nanog, and Sox2 form the core regulatory network that ensures the suppression of genes that lead to differentiation and the maintenance of pluripotency.[12] The cell surface antigens most commonly used to identify hES cells are the glycolipids stage specific embryonic antigen 3 and 4 and the keratan sulfate antigens Tra-1-60 and Tra-1-81. The molecular definition of a stem cell includes many more proteins and continues to be a topic of research.[13]
There are currently no approved treatments using embryonic stem cells. The first human trial was approved by the US Food and Drug Administration in January 2009.[14] However, the human trial was not initiated until October 13, 2010 in Atlanta for spinal injury victims. On November 14, 2011 the company conducting the trial announced that it will discontinue further development of its stem cell programs.[15] ES cells, being pluripotent cells, require specific signals for correct differentiation—if injected directly into another body, ES cells will differentiate into many different types of cells, causing a teratoma. Differentiating ES cells into usable cells while avoiding transplant rejection are just a few of the hurdles that embryonic stem cell researchers still face.[16] Many nations currently have moratoria on either ES cell research or the production of new ES cell lines. Because of their combined abilities of unlimited expansion and pluripotency, embryonic stem cells remain a theoretically potential source for regenerative medicine and tissue replacement after injury or disease.
Fetal
The primitive stem cells located in the organs of fetuses are referred to as fetal stem cells.[17] There are two types of fetal stem cells:- Fetal proper stem cells come from the tissue of the fetus proper, and are generally obtained after an abortion. These stem cells are not immortal but have a high level of division and are multipotent.
- Extraembryonic fetal stem cells come from extraembryonic membranes, and are generally not distinguished from adult stem cells. These stem cells are acquired after birth, they are not immortal but have a high level of cell division, and are pluripotent.[18]
Adult

Adult stem cells, also called somatic (from Greek Σωματικóς, "of the body") stem cells, are stem cells which maintain and repair the tissue in which they are found.[19] They can be found in children, as well as adults.[20]
Pluripotent adult stem cells are rare and generally small in number, but they can be found in umbilical cord blood and other tissues.[21] Bone marrow is a rich source of adult stem cells,[22] which have been used in treating several conditions including spinal cord injury,[23] liver cirrhosis,[24] chronic limb ischemia [25] and end stage heart failure.[26] The quantity of bone marrow stem cells declines with age and is greater in males than females during reproductive years.[27] Much adult stem cell research to date has aimed to characterize their potency and self-renewal capabilities.[28] DNA damage accumulates with age in both stem cells and the cells that comprise the stem cell environment. This accumulation is considered to be responsible, at least in part, for increasing stem cell dysfunction with aging (see DNA damage theory of aging).[29]
Most adult stem cells are lineage-restricted (multipotent) and are generally referred to by their tissue origin (mesenchymal stem cell, adipose-derived stem cell, endothelial stem cell, dental pulp stem cell, etc.).[30][31]
Adult stem cell treatments have been successfully used for many years to treat leukemia and related bone/blood cancers through bone marrow transplants.[32] Adult stem cells are also used in veterinary medicine to treat tendon and ligament injuries in horses.[33]
The use of adult stem cells in research and therapy is not as controversial as the use of embryonic stem cells, because the production of adult stem cells does not require the destruction of an embryo. Additionally, in instances where adult stem cells are obtained from the intended recipient (an autograft), the risk of rejection is essentially non-existent. Consequently, more US government funding is being provided for adult stem cell research.[34]
Amniotic
Multipotent stem cells are also found in amniotic fluid. These stem cells are very active, expand extensively without feeders and are not tumorigenic. Amniotic stem cells are multipotent and can differentiate in cells of adipogenic, osteogenic, myogenic, endothelial, hepatic and also neuronal lines.[35] Amniotic stem cells are a topic of active research.Use of stem cells from amniotic fluid overcomes the ethical objections to using human embryos as a source of cells. Roman Catholic teaching forbids the use of embryonic stem cells in experimentation; accordingly, the Vatican newspaper "Osservatore Romano" called amniotic stem cells "the future of medicine".[36]
It is possible to collect amniotic stem cells for donors or for autologuous use: the first US amniotic stem cells bank [37][38] was opened in 2009 in Medford, MA, by Biocell Center Corporation[39][40][41] and collaborates with various hospitals and universities all over the world.[42]
Cord blood
A certain kind of cord blood stem cell (CB-SC) is multipotent and displays embryonic and hematopoietic characteristics. Phenotypic characterization demonstrates that (CB-SCs) display embryonic cell markers (e.g., transcription factors OCT-4 and Nanog, stage-specific embryonic antigen (SSEA)-3, and SSEA-4) and leukocyte common antigen CD45, but that they are negative for blood cell lineage markers (e.g., CD1a, CD3, CD4, CD8, CD11b, CD11c, CD13, CD14, CD19, CD20, CD34, CD41a, CD41b, CD83, CD90, CD105, and CD133).[43][44]Additionally, CB-SCs display very low immunogenicity as indicated by expression of a very low level of major histocompatibility complex (MHC) antigens and failure to stimulate the proliferation of allogeneic lymphocytes.[43][45] They can give rise to three embryonic layer-derived cells in the presence of different inducers.[43][46]
More specifically, CB-SCs tightly adhere to culture dishes with a large rounded morphology and are resistant to common detaching methods (trypsin/EDTA).[43][45][46] CB-SCs are the active agent in stem cell educator therapy, which has therapeutic potential against autoimmune diseases like type 1 diabetes according to studies by Yong Zhao et al.[44][47][48][49][unreliable medical source?]
Induced pluripotent
These are not adult stem cells, but rather adult cells (e.g. epithelial cells) reprogrammed to give rise to pluripotent capabilities. Using genetic reprogramming with protein transcription factors, pluripotent stem cells equivalent to embryonic stem cells have been derived from human adult skin tissue.[50][51][52] Shinya Yamanaka and his colleagues at Kyoto University used the transcription factors Oct3/4, Sox2, c-Myc, and Klf4[50] in their experiments on cells from human faces. Junying Yu, James Thomson, and their colleagues at the University of Wisconsin–Madison used a different set of factors, Oct4, Sox2, Nanog and Lin28,[50] and carried out their experiments using cells from human foreskin.
As a result of the success of these experiments, Ian Wilmut, who helped create the first cloned animal Dolly the Sheep, has announced that he will abandon somatic cell nuclear transfer as an avenue of research.[53]
Frozen blood samples can be used as a source of induced pluripotent stem cells, opening a new avenue for obtaining the valued cells.[54]
An alternative theory is that stem cells remain undifferentiated due to environmental cues in their particular niche. Stem cells differentiate when they leave that niche or no longer receive those signals. Studies in Drosophila germarium have identified the signals decapentaplegic and adherens junctions that prevent germarium stem cells from differentiating.[56][57]

Diseases and conditions where stem cell treatment is being investigated include:
Research is underway to develop various sources for stem cells, and to apply stem cell treatments for neurodegenerative diseases and conditions, diabetes, heart disease, and other conditions.[73]
In more recent years, with the ability of scientists to isolate and culture embryonic stem cells, and with scientists' growing ability to create stem cells using somatic cell nuclear transfer and techniques to created induced pluripotent stem cells, controversy has crept in, both related to abortion politics and to human cloning.
Pluripotency in certain stem cells could also make it difficult to obtain a specific cell type. It is also difficult to obtain the exact cell type needed, because not all cells in a population differentiate uniformly. Undifferentiated cells can create tissues other than desired types.[74]
Some stem cells form tumors after transplantation; pluripotency is linked to tumor formation especially in embryonic stem cells, fetal proper stem cells, induced pluripotent stem cells. Fetal proper stem cells form tumors despite multipotency.[citation needed]
Hepatotoxicity and drug-induced liver injury account for a substantial number of failures of new drugs in development and market withdrawal, highlighting the need for screening assays such as stem cell-derived hepatocyte-like cells, that are capable of detecting toxicity early in the drug development process.[75]
Some of the fundamental patents covering human embryonic stem cells are owned by the Wisconsin Alumni Research Foundation (WARF) - they are patents 5,843,780, 6,200,806, and 7,029,913 invented by James A. Thomson. WARF does not enforce these patents against academic scientists, but does enforce them against companies.[76]
In 2006, a request for the US Patent and Trademark Office (USPTO) to re-examine the three patents was filed by the Public Patent Foundation on behalf of its client, the non-profit patent-watchdog group Consumer Watchdog (formerly the Foundation for Taxpayer and Consumer Rights).[76] In the re-examination process, which involves several rounds of discussion between the USTPO and the parties, the USPTO initially agreed with Consumer Watchdog and rejected all the claims in all three patents,[77] however in response, WARF amended the claims of all three patents to make them more narrow, and in 2008 the USPTO found the amended claims in all three patents to be patentable. The decision on one of the patents (7,029,913) was appealable, while the decisions on the other two were not.[78][79] Consumer Watchdog appealed the granting of the '913 patent to the USTPO's Board of Patent Appeals and Interferences (BPAI) which granted the appeal, and in 2010 the BPAI decided that the amended claims of the '913 patent were not patentable.[80] However, WARF was able to re-open prosecution of the case and did so, amending the claims of the '913 patent again to make them more narrow, and in January 2013 the amended claims were allowed.[81]
In July 2013, Consumer Watchdog announced that it would appeal the decision to allow the claims of the '913 patent to the US Court of Appeals for the Federal Circuit (CAFC), the federal appeals court that hears patent cases.[82] At a hearing in December 2013, the CAFC raised the question of whether Consumer Watchdog had legal standing to appeal; the case could not proceed until that issue was resolved.[83]
As a result of the success of these experiments, Ian Wilmut, who helped create the first cloned animal Dolly the Sheep, has announced that he will abandon somatic cell nuclear transfer as an avenue of research.[53]
Frozen blood samples can be used as a source of induced pluripotent stem cells, opening a new avenue for obtaining the valued cells.[54]
Lineage
To ensure self-renewal, stem cells undergo two types of cell division (see Stem cell division and differentiation diagram). Symmetric division gives rise to two identical daughter cells both endowed with stem cell properties. Asymmetric division, on the other hand, produces only one stem cell and a progenitor cell with limited self-renewal potential. Progenitors can go through several rounds of cell division before terminally differentiating into a mature cell. It is possible that the molecular distinction between symmetric and asymmetric divisions lies in differential segregation of cell membrane proteins (such as receptors) between the daughter cells.[55]An alternative theory is that stem cells remain undifferentiated due to environmental cues in their particular niche. Stem cells differentiate when they leave that niche or no longer receive those signals. Studies in Drosophila germarium have identified the signals decapentaplegic and adherens junctions that prevent germarium stem cells from differentiating.[56][57]
Treatments

Diseases and conditions where stem cell treatment is being investigated include:
- Diabetes[58]
- Rheumatoid arthritis[58]
- Parkinson's disease[58]
- Alzheimer's disease[58]
- Osteoarthritis[58]
- Stroke and traumatic brain injury repair[59]
- Learning disability due to congenital disorder [60]
- Spinal cord injury repair [61]
- Heart infarction [62]
- Anti-cancer treatments [63]
- Baldness reversal[64]
- Replace missing teeth [65]
- Repair hearing [66]
- Restore vision [67]
- Amyotrophic lateral sclerosis [68]
- Crohn's disease [69]
- Wound healing [70]
Research is underway to develop various sources for stem cells, and to apply stem cell treatments for neurodegenerative diseases and conditions, diabetes, heart disease, and other conditions.[73]
In more recent years, with the ability of scientists to isolate and culture embryonic stem cells, and with scientists' growing ability to create stem cells using somatic cell nuclear transfer and techniques to created induced pluripotent stem cells, controversy has crept in, both related to abortion politics and to human cloning.
Disadvantages
Stem cell treatments may require immunosuppression because of a requirement for radiation before the transplant to remove the patient's previous cells, or because the patient's immune system may target the stem cells. One approach to avoid the second possibility is to use stem cells from the same patient who is being treated.Pluripotency in certain stem cells could also make it difficult to obtain a specific cell type. It is also difficult to obtain the exact cell type needed, because not all cells in a population differentiate uniformly. Undifferentiated cells can create tissues other than desired types.[74]
Some stem cells form tumors after transplantation; pluripotency is linked to tumor formation especially in embryonic stem cells, fetal proper stem cells, induced pluripotent stem cells. Fetal proper stem cells form tumors despite multipotency.[citation needed]
Hepatotoxicity and drug-induced liver injury account for a substantial number of failures of new drugs in development and market withdrawal, highlighting the need for screening assays such as stem cell-derived hepatocyte-like cells, that are capable of detecting toxicity early in the drug development process.[75]
Research patents
Further information: Consumer Watchdog vs. Wisconsin Alumni Research Foundation
Some of the fundamental patents covering human embryonic stem cells are owned by the Wisconsin Alumni Research Foundation (WARF) - they are patents 5,843,780, 6,200,806, and 7,029,913 invented by James A. Thomson. WARF does not enforce these patents against academic scientists, but does enforce them against companies.[76]
In 2006, a request for the US Patent and Trademark Office (USPTO) to re-examine the three patents was filed by the Public Patent Foundation on behalf of its client, the non-profit patent-watchdog group Consumer Watchdog (formerly the Foundation for Taxpayer and Consumer Rights).[76] In the re-examination process, which involves several rounds of discussion between the USTPO and the parties, the USPTO initially agreed with Consumer Watchdog and rejected all the claims in all three patents,[77] however in response, WARF amended the claims of all three patents to make them more narrow, and in 2008 the USPTO found the amended claims in all three patents to be patentable. The decision on one of the patents (7,029,913) was appealable, while the decisions on the other two were not.[78][79] Consumer Watchdog appealed the granting of the '913 patent to the USTPO's Board of Patent Appeals and Interferences (BPAI) which granted the appeal, and in 2010 the BPAI decided that the amended claims of the '913 patent were not patentable.[80] However, WARF was able to re-open prosecution of the case and did so, amending the claims of the '913 patent again to make them more narrow, and in January 2013 the amended claims were allowed.[81]
In July 2013, Consumer Watchdog announced that it would appeal the decision to allow the claims of the '913 patent to the US Court of Appeals for the Federal Circuit (CAFC), the federal appeals court that hears patent cases.[82] At a hearing in December 2013, the CAFC raised the question of whether Consumer Watchdog had legal standing to appeal; the case could not proceed until that issue was resolved.[83]
Key research events
- 1908: The term "stem cell" was proposed for scientific use by the Russian histologist Alexander Maksimov (1874–1928) at congress of hematologic society in Berlin. It postulated existence of haematopoietic stem cells.
- 1960s: Joseph Altman and Gopal Das present scientific evidence of adult neurogenesis, ongoing stem cell activity in the brain; their reports contradict Cajal's "no new neurons" dogma and are largely ignored.
- 1963: Becker, McCulloch and Till illustrate the presence of self-renewing cells in mouse bone marrow.
- 1968: Bone marrow transplant between two siblings successfully treats SCID.
- 1978: Haematopoietic stem cells are discovered in human cord blood.
- 1981: Mouse embryonic stem cells are derived from the inner cell mass by scientists Martin Evans, Matthew Kaufman, and Gail R. Martin. Gail Martin is attributed for coining the term "Embryonic Stem Cell".[84]
- 1992: Neural stem cells are cultured in vitro as neurospheres.
- 1995: Indian scientist Dr. B.G. Matapurkar pioneers in adult stem-cell research with clinical utilization of research in the body and neo-regeneration of tissues and organs in the body. Received International Patent from US Patent Office (USA) in 2001 (effective from 1995). Clinical utilization in human body also demonstrated and patented in 60 patients (World Journal of Surgery-1999[85] and 1991[86]).
- 1997: Dr. B.G. Matapurkar's surgical technique on regeneration of tissues and organs is published.[87] Regeneration of fallopian tube and uterus is published.[88]
- 1997: Leukemia is shown to originate from a haematopoietic stem cell, the first direct evidence for cancer stem cells.
- 1998: James Thomson and coworkers derive the first human embryonic stem cell line at the University of Wisconsin–Madison.[89]
- 1998: John Gearhart (Johns Hopkins University) extracted germ cells from fetal gonadal tissue (primordial germ cells) before developing pluripotent stem cell lines from the original extract.
- 2000s: Several reports of adult stem cell plasticity are published.
- 2001: Scientists at Advanced Cell Technology clone first early (four- to six-cell stage) human embryos for the purpose of generating embryonic stem cells.[90]
- 2003: Dr. Songtao Shi of NIH discovers new source of adult stem cells in children's primary teeth.[91]
- 2004–2005: Korean researcher Hwang Woo-Suk claims to have created several human embryonic stem cell lines from unfertilised human oocytes. The lines were later shown to be fabricated.
- 2005: Researchers at Kingston University in England claim to have discovered a third category of stem cell, dubbed cord-blood-derived embryonic-like stem cells (CBEs), derived from umbilical cord blood. The group claims these cells are able to differentiate into more types of tissue than adult stem cells.
- 2005: Researchers at UC Irvine's Reeve-Irvine Research Center are able to partially restore the ability of rats with paralyzed spines to walk through the injection of human neural stem cells.[92]
- April 2006 Scientists at the University of Illinois at Chicago identified novel stem cells from the umbilical cord blood with embryonic and hematopoietic characteristics.[43]
- August 2006: Kazutoshi Takahashi and Shinya Yamanaka publish evidence of Induced pluripotent stem cells in mice in the journal Cell.[93]
- November 2006: Yong Zhao et al. revealed the immune regulation of T lymphocytes by Cord Blood-Derived Multipotent Stem Cells (CB-SCs).[45]
- October 2006: Scientists at Newcastle University in England create the first ever artificial liver cells using umbilical cord blood stem cells.[94][95]
- January 2007: Scientists at Wake Forest University led by Dr. Anthony Atala and Harvard University report discovery of a new type of stem cell in amniotic fluid.[96] This may potentially provide an alternative to embryonic stem cells for use in research and therapy.[97]
- June 2007: Research reported by three different groups shows that normal skin cells can be reprogrammed to an embryonic state in mice.[98] In the same month, scientist Shoukhrat Mitalipov reports the first successful creation of a primate stem cell line through somatic cell nuclear transfer[99]
- October 2007: Mario Capecchi, Martin Evans, and Oliver Smithies win the 2007 Nobel Prize for Physiology or Medicine for their work on embryonic stem cells from mice using gene targeting strategies producing genetically engineered mice (known as knockout mice) for gene research.[100]
- November 2007: Human induced pluripotent stem cells: Two similar papers released by their respective journals prior to formal publication: in Cell by Kazutoshi Takahashi and Shinya Yamanaka, "Induction of pluripotent stem cells from adult human fibroblasts by defined factors",[101] and in Science by Junying Yu, et al., from the research group of James Thomson, "Induced pluripotent stem cell lines derived from human somatic cells":[102] pluripotent stem cells generated from mature human fibroblasts. It is possible now to produce a stem cell from almost any other human cell instead of using embryos as needed previously, albeit the risk of tumorigenesis due to c-myc and retroviral gene transfer remains to be determined.
- January 2008: Robert Lanza and colleagues at Advanced Cell Technology and UCSF create the first human embryonic stem cells without destruction of the embryo.[103]
- January 2008: Development of human cloned blastocysts following somatic cell nuclear transfer with adult fibroblasts[104]
- February 2008: Generation of pluripotent stem cells from adult mouse liver and stomach: these iPS cells seem to be more similar to embryonic stem cells than the previously developed iPS cells and not tumorigenic, moreover genes that are required for iPS cells do not need to be inserted into specific sites, which encourages the development of non-viral reprogramming techniques.[105]
- March 2008-The first published study of successful cartilage regeneration in the human knee using autologous adult mesenchymal stem cells is published by clinicians from Regenerative Sciences[106]
- October 2008: Sabine Conrad and colleagues at Tübingen, Germany generate pluripotent stem cells from spermatogonial cells of adult human testis by culturing the cells in vitro under leukemia inhibitory factor (LIF) supplementation.[107]
- 30 October 2008: Embryonic-like stem cells from a single human hair.[108]
- January 2009: Yong Zhao and colleagues confirmed the reversal of autoimmune-caused type 1 diabetes by Cord Blood-Derived Multipotent Stem Cells (CB-SCs) in an animal experiment.[44][47]
- 1 March 2009: Andras Nagy, Keisuke Kaji, et al. discover a way to produce embryonic-like stem cells from normal adult cells by using a novel "wrapping" procedure to deliver specific genes to adult cells to reprogram them into stem cells without the risks of using a virus to make the change.[109][110][111] The use of electroporation is said to allow for the temporary insertion of genes into the cell.[112][112][113][114]
- 28 May 2009 Kim et al. announced that they had devised a way to manipulate skin cells to create patient specific "induced pluripotent stem cells" (iPS), claiming it to be the 'ultimate stem cell solution'.[115]
- 11 October 2010 First trial of embryonic stem cells in humans.[116]
- 25 October 2010: Ishikawa et al. write in the Journal of Experimental Medicine that research shows that transplanted cells that contain their new host's nuclear DNA could still be rejected by the individual's immune system due to foreign mitochondrial DNA. Tissues made from a person's stem cells could therefore be rejected, because mitochondrial genomes tend to accumulate mutations.[117]
- 2011: Israeli scientist Inbar Friedrich Ben-Nun led a team which produced the first stem cells from endangered species, a breakthrough that could save animals in danger of extinction.[118]
- January 2012: The human clinical trial of treating type 1 diabetes with lymphocyte modification using Cord Blood-Derived Multipotent Stem Cells (CB-SCs) achieved an improvement of C-peptide levels, reduced the median glycated hemoglobin A1C (HbA1c) values, and decreased the median daily dose of insulin in both human patient groups with and without residual beta cell function.[48][49] Yong Zhao's Stem Cell Educator Therapy appears "so simple and so safe"[119]
- October 2012: Positions of nucleosomes in mouse embryonic stem cells and the changes in their positions during differentiation to neural progenitor cells and embryonic fibroblasts are determined with single-nucleotide resolution.[120]
- 2012: Katsuhiko Hayashi used mouse skin cells to create stem cells and then used these stem cells to create mouse eggs. These eggs were then fertilized and produced healthy baby offspring. These latter mice were able to have their own babies.[121]
- 2013: First time lab grown meat made from muscle stem-cells has been cooked and tasted.[122]
- 2013: First time mice adult cells were reprogrammed into stem cells in vivo.[123]
- 2013: Scientists at Scotland's Heriot-Watt University developed a 3D printer that can produce clusters of living human embryonic stem cells, potentially allowing complete organs to be printed on demand in the future.[124]
- 2014: Adult mouse cells reprogrammed to pluripotent stem cells using stimulus-triggered acquisition of pluripotency (STAP);[125] a process which involved bathing blood cells in an acid bath (pH 5.7) for 30minutes at 37 °C.[126] A little over a month after the publication of these findings, errors were discovered and the quality of the research has been widely questioned.[127] Further irregularities regarding the mice used have emerged as recently as June 2014.[128]