From Wikipedia, the free encyclopedia
Senescence (
) or
biological aging (also spelled
biological aging) is the gradual deterioration of
function characteristic of most complex lifeforms, arguably found in all
biological kingdoms, that on the level of the
organism increases
mortality after
maturation. The word
senescence can refer either to
cellular senescence
or to senescence of the whole organism. It is commonly believed that
cellular senescence underlies organismal senescence. The science of
biological aging is
biogerontology.
Senescence
is not the inevitable fate of all organisms and can be delayed. The discovery, in 1934, that
calorie restriction can extend lifespan by 50% in rats, and the existence of species having
negligible senescence and potentially immortal species such as
Hydra, have motivated research into delaying and preventing senescence and thus age-related diseases. Organisms of some
taxonomic groups, including some
animals, experience chronological decrease in mortality, for all or part of their life cycle.
[1] On the other extreme are
accelerated aging diseases, rare in humans. There is also the extremely rare and poorly understood "
Syndrome X," whereby a person remains physically and mentally an infant or child throughout one's life.
[2][3]
Even if environmental factors do not cause aging, they may affect it; in such a way, for example, overexposure to
ultraviolet radiation accelerates
skin aging.
Different parts of the body may age at different rates. Two organisms
of the same species can also age at different rates, so that biological
aging and chronological aging are quite distinct concepts.
Albeit indirectly, senescence is by far the leading cause of death (other than in the trivially accurate sense that
cerebral hypoxia,
i.e.,
lack of oxygen to the brain, is the immediate cause of all human
death). Of the roughly 150,000 people who die each day across the globe,
about two thirds – 100,000 per day – die of age-related causes; in
industrialized nations, moreover, the proportion is much higher,
reaching 90%.
[4]
There are a number of hypotheses as to why senescence occurs; for example, some posit it is programmed by
gene expression
changes, others that it is the cumulative damage caused by biological
processes. Whether senescence as a biological process itself can be
slowed down, halted or even reversed, is a subject of current scientific
speculation and research.
[5]
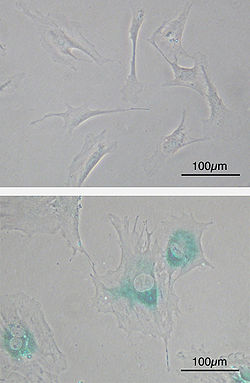
Cellular senescence or cellular aging
Cellular senescence
(upper) Primary mouse embryonic fibroblast cells (MEFs) before
senescence. Spindle-shaped. (lower) MEFs became senescent after
passages. Cells grow larger, flatten shape and expressed
senescence-associated
β-galactosidase (SABG, blue areas), a marker of cellular senescence.
Cellular senescence is the phenomenon by which normal
diploid cells cease to
divide.
In culture, fibroblasts can reach a maximum of 50 cell divisions before
becoming senescent. This phenomenon is known as "replicative
senescence", or the
Hayflick limit.
[6] Replicative senescence is the result of
telomere shortening that ultimately triggers a
DNA damage response. Cells can also be induced to senesce via DNA damage in response to elevated
reactive oxygen species (ROS), activation of
oncogenes and cell-
cell fusion,
independent of telomere length. As such, cellular senescence represents
a change in "cell state" rather than a cell becoming "aged" as the name
confusingly suggests.
Although senescent cells can no longer replicate, they remain metabolically active and commonly adopt an immunogenic
phenotype consisting of a pro-inflammatory secretome, the up-regulation of immune
ligands, a pro-survival response, promiscuous gene expression (pGE) and stain positive for
senescence-associated β-galactosidase activity.
[7] The nucleus of senescent cells is characterized by senescence-associated
heterochromatin foci (SAHF) and
DNA segments with chromatin alterations reinforcing senescence (DNA-SCARS).
[8]
Senescent cells affect tumour suppression, wound healing and possibly
embryonic/placental development and a pathological role in age-related
diseases.
[9]
The experimental elimination of senescent cells from transgenic
progeroid mice
[10] and non-progeroid, naturally-aged mice
[11][12][13] led to greater resistance against
aging-associated diseases.
Ectopic expression of the embryonic transcription factor, NANOG, is
shown to reverse senescence and restore the proliferation and
differentiation potential of senescent stem cells.
[14][15][16][17][18]
Epigenetic clock analysis of cellular senescence
According to a molecular biomarker of aging known as
epigenetic clock,
[19]
the three major types of cellular senescence, namely replicative
senescence, oncogene-induced senescence and DNA damage-induced
senescence are distinct because induction of replicative senescence (RS)
and oncogene-induced senescence (OIS) were found to be accompanied by
epigenetic aging of primary cells but senescence induced by DNA damage
was not, even though RS and OIS activate the cellular DNA damage
response pathway.
[20]
These results highlight the independence of cellular senescence from
epigenetic aging. Consistent with this, telomerase-immortalised cells
continued to age (according to the epigenetic clock) without having been
treated with any senescence inducers or DNA-damaging agents,
re-affirming the independence of the process of epigenetic ageing from
telomeres, cellular senescence, and the DNA damage response pathway.
Although the uncoupling of senescence from cellular aging appears at
first sight to be inconsistent with the fact that senescent cells
contribute to the physical manifestation of organism ageing, as
demonstrated by Baker et al., where removal of senescent cells slowed
down aging.
[10]
However, the epigenetic clock analysis of senescence suggests that
cellular senescence is a state that cells are forced into as a result of
external pressures such as DNA damage, ectopic oncogene expression and
exhaustive proliferation of cells to replenish those eliminated by
external/environmental factors.
[20]
These senescent cells, in sufficient numbers, will undoubtedly cause
the deterioration of tissues, which is interpreted as organism ageing. However, at the cellular level, aging, as measured by the epigenetic
clock, is distinct from senescence. It is an intrinsic mechanism that
exists from the birth of the cell and continues. This implies that if
cells are not shunted into senescence by the external pressures
described above, they would still continue to age. This is consistent
with the fact that mice with naturally long telomeres still age and
eventually die even though their telomere lengths are far longer than
the critical limit, and they age prematurely when their telomeres are
forcibly shortened, due to replicative senescence. Hence senescence is a
route by which cells exit prematurely from the natural course of
cellular ageing.
[20]
Aging of the whole organism
Organismal senescence is the aging of whole organisms. In general,
aging is characterized by the declining ability to respond to stress, increased
homeostatic imbalance, and increased risk of
aging-associated diseases.
Death is the ultimate consequence of aging, though "old age" is not a scientifically recognized
cause of death because there is always a specific proximal cause, such as
cancer,
heart disease, or
liver failure.
Aging of whole organisms is therefore a complex process that can be
defined as "a progressive deterioration of physiological function, an
intrinsic age-related process of loss of viability and increase in
vulnerability."
[21]
Differences in
maximum life span among species correspond to different "rates of aging." For example,
inherited differences in the rate of aging make a
mouse elderly at 3 years and a
human elderly at 80 years.
[22] These genetic differences affect a variety of physiological processes, including the efficiency of
DNA repair,
antioxidant enzymes, and rates of
free radical production.
Supercentenarian Ann Pouder (8 April 1807 – 10 July 1917) photographed on her 110th birthday. A heavily lined face is common in human senescence.
Senescence of the organism gives rise to the
Gompertz–Makeham law of mortality, which says that
mortality rate accelerates rapidly with age.
Some animals, such as some reptiles and fish, age slowly (
negligible senescence)
and exhibit very long lifespans. Some even exhibit "negative
senescence", in which mortality falls with age, in disagreement with the
Gompertz–Makeham "law".
[1]
Whether replicative senescence (
Hayflick limit) plays a causative role in organismal aging is at present an active area of investigation.
The oft-quoted evolutionary theorist George Williams wrote, "It is remarkable that after a seemingly miraculous feat of
morphogenesis, a complex
metazoan should be unable to perform the much simpler task of merely maintaining what is already formed."
[23]
There is a current debate as to whether or not the pursuit of
longevity
and the postponement of senescence are cost-effective health care goals
given finite health care resources. Because of the accumulated
infirmities of old age, bioethicist
Ezekiel Emanuel, opines that the pursuit of longevity via the
compression of morbidity
hypothesis is a "fantasy" and that human life is not worth living after
age 75; longevity then should not be a goal of health care policy.
[24] This opinion has been contested by neurosurgeon and medical ethicist
Miguel Faria,
who states that life can be worthwhile during old age, and that
longevity should be pursued in association with the attainment of
quality of life.
[25]
Faria claims that postponement of senescence as well as happiness and
wisdom can be attained in old age in a large proportion of those who
lead healthy lifestyles and remain intellectually active.
[26]
Theories of aging
The
exact etiology of senescence is still largely unclear and yet to be
discovered. The process of senescence is complex, and may derive from a
variety of different mechanisms and exist for a variety of different
reasons. However, senescence is not universal. In a few simple species,
such as those in the genus
Hydra, senescence is negligible and cannot be detected.
Another related mechanism is that of the biologically immortal
planarian
flatworms, which have "apparently limitless [telomere] regenerative
capacity fueled by a population of highly proliferative adult
stem cells."
[27] These organisms are
biologically immortal but not immortal in the traditional sense as they are nonetheless susceptible to
trauma and
infectious and
non-infectious disease. Moreover, average lifespans can vary greatly within and between
species. This suggests that both
genetic and environmental factors contribute to aging.
In general, theories that explain senescence have been divided between the programmed and
stochastic
theories of aging. Programmed theories imply that aging is regulated by
biological clocks operating throughout the lifespan. This regulation
would depend on changes in
gene expression that affect the systems responsible for maintenance, repair, and defense responses. The
reproductive-cell cycle theory suggests that aging is caused by changes in hormonal signaling over the lifespan.
[28]
Stochastic theories blame environmental impacts on living organisms
that induce cumulative damage at various levels as the cause of aging,
examples of which ranging from
damage to DNA, damage to tissues and cells by oxygen
radicals (widely known as
free radicals countered by the even more well-known
antioxidants), and
cross-linking.
However, aging is seen as a progressive failure of homeodynamics–systemic preservation of
homeostasis,
involving genes for maintenance and repair, stochastic events leading
to molecular damage and molecular heterogeneity, and chance events
determining the probability of death. Since complex and interacting
systems of maintenance and repair comprise the homeodynamic space of a
biological system, aging is considered to be a progressive shrinkage of
homeodynamic space mainly due to increased molecular heterogeneity.
[citation needed]
In 2013, a group of scientists defined nine hallmarks of aging that are
common between organisms with emphasis on mammals: genomic instability,
telomere attrition, epigenetic alterations, loss of
proteostasis,
deregulated nutrient sensing, mitochondrial dysfunction, cellular
senescence, stem cell exhaustion, and altered intercellular
communication.
[29]
Evolutionary theories
A gene can be expressed at various stages of life. Therefore, natural
selection can support lethal and harmful alleles, if their expression
occurs after reproduction. Senescence may be the product of such
selection.
[30][31][32]
In addition, ageing is believed to have evolved because of the
increasingly smaller probability of an organism still being alive at
older age, due to predation and accidents, both of which may be random
and age-invariant. The antagonistic plietropy theory states that
strategies which result in a higher reproductive rate at a young age,
but shorter overall lifespan, result in a higher lifetime reproductive
success and are therefore favoured by
natural selection.
In essence, aging is, therefore, the result of investing resources in
reproduction, rather than maintenance of the body (the "Disposable Soma"
theory
[33]),
in light of the fact that accidents, predation, and disease kill
organisms regardless of how much energy is devoted to repair of the
body. Various other theories of aging exist, and are not necessarily
mutually exclusive.
The geneticist
J. B. S. Haldane wondered why the dominant mutation that causes
Huntington's disease
remained in the population, and why natural selection had not
eliminated it. The onset of this neurological disease is (on average) at
age 45 and is invariably fatal within 10–20 years. Haldane assumed
that, in human prehistory, few survived until age 45. Since few were
alive at older ages and their contribution to the next generation was
therefore small relative to the large cohorts of younger age groups, the
force of selection against such late-acting deleterious mutations was
correspondingly small. However, if a mutation affected younger
individuals, selection against it would be strong. Therefore,
late-acting deleterious mutations could accumulate in populations over
evolutionary time through
genetic drift,
which has been demonstrated experimentally. This concept of higher
accumulation of deleterious mutations for older organisms came to be
known as the
selection shadow.
[34]
Peter Medawar formalised this observation in his
mutation accumulation theory of aging.
[35][36]
"The force of natural selection weakens with increasing age—even in a
theoretically immortal population, provided only that it is exposed to
real hazards of mortality. If a genetic disaster... happens late enough
in individual life, its consequences may be completely unimportant". The
'real hazards of mortality' are, in typical circumstances, predation,
disease, and accidents. So, even an immortal population, whose fertility
does not decline with time, will have fewer individuals alive in older
age groups. This is called '
extrinsic mortality'.
Young cohorts, not depleted in numbers yet by extrinsic mortality,
contribute far more to the next generation than the few remaining older
cohorts, so the force of selection against late-acting deleterious
mutations, which affect only these few older individuals, is very weak.
The mutations may not be selected against, therefore, and may spread
over evolutionary time into the population.
The major testable prediction made by this model is that species that
have high extrinsic mortality in nature will age more quickly and have
shorter
intrinsic lifespans.
This is borne out among mammals, the best-studied in terms of life
history. There is a correlation among mammals between body size and
lifespan,
such that larger species live longer than smaller species under
controlled/optimum conditions, but there are notable exceptions. For
instance, many bats and rodents are of similar size, yet bats live much
longer. For instance, the
little brown bat, half the size of a
mouse,
can live 30 years in the wild. A mouse will only live 2–3 years even
under optimum conditions. The explanation is that bats have fewer
predators, and therefore low extrinsic mortality. More individuals
survive to later ages, so the force of selection against late-acting
deleterious mutations is stronger. Fewer late-acting deleterious
mutations equates to slower aging and therefore a longer lifespan. Birds
are also warm-blooded and are similar in size to many small mammals,
yet often live 5–10 times as long. They have less predation pressure
than ground-dwelling mammals.
Seabirds, which, in general, have the fewest predators of all birds, live longest.
When examining the body-size vs. lifespan relationship, one also
observes that predatory mammals tend to live longer than prey mammals in
a controlled environment, such as a zoo or nature reserve. The
explanation for the long lifespans of primates (such as humans, monkeys,
and apes) relative to body size is that their intelligence, and often
their sociality, help them avoid becoming prey. High position in the
food chain, intelligence and cooperativeness all reduce extrinsic mortality in species.
Another evolutionary theory of aging was proposed by
George C. Williams[37] and involves
antagonistic pleiotropy.
A single gene may affect multiple traits. Some traits that increase
fitness early in life may also have negative effects later in life. But,
because many more individuals are alive at young ages than at old ages,
even small positive effects early can be strongly selected for, and
large negative effects later may be very weakly selected against.
Williams suggested the following example: Perhaps a gene codes for
calcium deposition in bones, which promotes juvenile survival and will
therefore be favored by natural selection; however, this same gene
promotes calcium deposition in the arteries, causing negative
atherosclerotic effects in old age. Thus, harmful biological changes in
old age may result from selection for
pleiotropic genes that are beneficial early in life but harmful later on. In this case, selection pressure is relatively high when
Fisher's reproductive value is high and relatively low when Fisher's reproductive value is low.
Gene regulation
A number of genetic components of aging have been identified using model organisms, ranging from the simple budding
yeast Saccharomyces cerevisiae to worms such as
Caenorhabditis elegans and
fruit flies (
Drosophila melanogaster). Study of these organisms has revealed the presence of at least two conserved aging pathways.
One of these pathways involves the gene
Sir2, a
NAD+-dependent histone deacetylase. In yeast, Sir2 is required for genomic silencing at three loci: the yeast mating
loci, the
telomeres and the
ribosomal DNA (rDNA). In some species of yeast, replicative aging may be partially caused by
homologous recombination between rDNA repeats;
excision of rDNA repeats results in the formation of
extrachromosomal rDNA circles
(ERCs). These ERCs replicate and preferentially segregate to the mother
cell during cell division, and are believed to result in cellular
senescence by
titrating away (competing for) essential
nuclear factors.
ERCs have not been observed in other species (nor even all strains of
the same yeast species) of yeast (which also display replicative
senescence), and ERCs are not believed to contribute to aging in higher
organisms such as humans (they have not been shown to accumulate in
mammals in a similar manner to yeast). Extrachromosomal circular DNA
(eccDNA) has been found in worms, flies, and humans. The origin and role
of eccDNA in aging, if any, is unknown.
Despite the lack of a connection between circular DNA and aging in
higher organisms, extra copies of Sir2 are capable of extending the
lifespan of both worms and flies (though, in flies, this finding has not
been replicated by other investigators, and the activator of Sir2
resveratrol does not reproducibly increase lifespan in either species.
[38])
Whether the Sir2 homologues in higher organisms have any role in
lifespan is unclear, but the human SIRT1 protein has been demonstrated
to
deacetylate p53, Ku70, and the
forkhead family of
transcription factors. SIRT1 can also regulate acetylates such as
CBP/p300, and has been shown to deacetylate specific
histone residues.
RAS1 and RAS2 also affect aging in yeast and have a human homologue.
RAS2 overexpression has been shown to extend lifespan in yeast.
Other genes regulate aging in yeast by increasing the resistance to
oxidative stress.
Superoxide dismutase, a
protein that protects against the effects of
mitochondrial free
radicals, can extend yeast lifespan in stationary phase when overexpressed.
In higher organisms, aging is likely to be regulated in part through the insulin/IGF-1 pathway. Mutations that affect
insulin-like signaling
in worms, flies, and the growth hormone/IGF1 axis in mice are
associated with extended lifespan. In yeast, Sir2 activity is regulated
by the nicotinamidase PNC1. PNC1 is transcriptionally
upregulated under stressful conditions such as
caloric restriction,
heat shock, and
osmotic shock. By converting
nicotinamide to
niacin, nicotinamide is removed, inhibiting the activity of Sir2. A
nicotinamidase found in humans, known as
PBEF, may serve a similar function, and a secreted form of PBEF known as
visfatin may help to regulate serum
insulin
levels. It is not known, however, whether these mechanisms also exist
in humans, since there are obvious differences in biology between humans
and model organisms.
Sir2 activity has been shown to increase under calorie restriction.
Due to the lack of available glucose in the cells, more NAD+ is
available and can activate Sir2.
Resveratrol, a stilbenoid found in the skin of red
grapes,
was reported to extend the lifespan of yeast, worms, and flies (the
lifespan extension in flies and worms have proved to be irreproducible
by independent investigators
[38]).
It has been shown to activate Sir2 and therefore mimics the effects of
calorie restriction, if one accepts that caloric restriction is indeed
dependent on Sir2.
Gene expression is imperfectly controlled, and it is possible that
random fluctuations in the expression levels of many genes contribute to
the aging process as suggested by a study of such genes in yeast.
[39]
Individual cells, which are genetically identical, none-the-less can
have substantially different responses to outside stimuli, and markedly
different lifespans, indicating the epigenetic factors play an important
role in gene expression and aging as well as genetic factors.
According to the GenAge database of aging-related genes there are over 700 genes associated with aging in
model organisms: 555 in the soil roundworm (
Caenorhabditis elegans), 87 in the bakers' yeast (
Saccharomyces cerevisiae), 75 in the fruit fly (
Drosophila melanogaster) and 68 in the mouse (
Mus musculus).
[40] The following is a list of genes connected to longevity through research
[40] on
model organisms:
Cellular senescence
As
noted above, senescence is not universal. It was once thought that
senescence did not occur in single-celled organisms that reproduce
through the process of cellular
mitosis.
[41]
Recent investigation has unveiled a more complex picture. Single cells
do accumulate age-related damage. On mitosis the debris is not evenly
divided between the new cells. Instead it passes to one of the cells
leaving the other cell pristine. With successive generations the cell
population becomes a mosaic of cells with half ageless and the rest with
varying degrees of senescence.
[42]
Moreover, cellular senescence is not observed in several organisms, including
perennial plants,
sponges,
corals, and
lobsters. In those species where cellular senescence is observed, cells eventually become post-
mitotic when they can no longer replicate themselves through the process of
cellular mitosis; i.e., cells experience
replicative senescence.
How and why some cells become post-mitotic in some species has been the
subject of much research and speculation, but (as noted above) it is
sometimes suggested that cellular senescence evolved as a way to prevent
the onset and spread of
cancer.
Somatic cells that have divided many times will have accumulated
DNA mutations and would therefore be in danger of becoming
cancerous
if cell division continued. As such, it is becoming apparent that
senescent cells undergo conversion to an immunogenic phenotype that
enables them to be eliminated by the immune system.
[43]
Lately, the role of
telomeres in cellular senescence has aroused general interest, especially with a view to the possible genetically adverse effects of
cloning. The successive shortening of the
chromosomal telomeres with each
cell cycle
is also believed to limit the number of divisions of the cell, thus
contributing to aging. There have, on the other hand, also been reports
that cloning could alter the shortening of telomeres. Some cells do not
age and are, therefore, described as being "
biologically immortal".
It is theorized by some that when it is discovered exactly what allows
these cells, whether it be the result of telomere lengthening or not, to
divide without limit that it will be possible to genetically alter
other cells to have the same capability. It is further theorized that it
will eventually be possible to
genetically engineer all cells in the human body to have this capability by employing
gene therapy and, therefore, stop or reverse aging, effectively making the entire organism potentially immortal.
The length of the telomere strand has senescent effects; telomere
shortening activates extensive alterations in alternative RNA splicing
that produce senescent toxins such as
progerin, which degrades the tissue and makes it more prone to failure.
[44]
Cancer
cells are usually immortal. In about 85% of tumors, this evasion of
cellular senescence is the result of up-activation of their
telomerase genes.
[45]
This simple observation suggests that reactivation of telomerase in
healthy individuals could greatly increase their cancer risk.
Ned Sharpless and collaborators demonstrated the first in vivo link between p16-expression and lifespan.
[46]
They found reduced p16 expression in some tissues of mice with
mutations that extend lifespan, as well as in mice that had their
lifespan extended by food restriction. Jan van Deursen and Darren Baker
in collaboration with Andre Terzic at the
Mayo Clinic
in Rochester, Minn., provided the first in vivo evidence for a causal
link between cellular senescence and aging by preventing the
accumulation of senescent cells in BubR1 progeroid mice.
[47]
In the absence of senescent cells, the mice’s tissues showed a major
improvement in the usual burden of age-related disorders. They did not
develop
cataracts,
avoided the usual wasting of muscle with age. They retained the fat
layers in the skin that usually thin out with age and, in people, cause
wrinkling. A second study led by Jan van Deursen in collaboration with a
team of collaborators at the Mayo Clinic and Groningen University,
provided the first direct in vivo evidence that cellular senescence
causes signs of aging by eliminating senescent cells from progeroid mice
by introducing a drug-inducible suicide gene and then treating the mice
with the drug to kill senescent cells selectively, as opposed to
decreasing whole body p16.
[10]
Another Mayo study led by James Kirkland in collaboration with Scripps
and other groups demonstrated that senolytics, drugs that target
senescent cells, enhance cardiac function and improve vascular
reactivity in old mice, alleviate gait disturbance caused by radiation
in mice, and delay frailty, neurological dysfunction, and osteoporosis
in progeroid mice. Discovery of senolytic drugs was based on a
hypothesis-driven approach: the investigators leveraged the observation
that senescent cells are resistant to apoptosis to discover that
pro-survival pathways are up-regulated in these cells. They demonstrated
that these survival pathways are the "Achilles heel" of senescent cells
using RNA interference approaches, including Bcl-2-, AKT-, p21-, and
tyrosine kinase-related pathways. They then used drugs known to target
the identified pathways and showed these drugs kill senescent cells by
apoptosis in culture and decrease senescent cell burden in multiple
tissues in vivo. Importantly, these drugs had long term effects after a
single dose, consistent with removal of senescent cells, rather than a
temporary effect requiring continued presence of the drugs. This was the
first study to show that clearing senescent cells enhances function in
chronologically aged mice.
[48]
Chemical damage
One of the earliest aging theories was the
Rate of Living Hypothesis described by
Raymond Pearl in 1928
[49] (based on earlier work by
Max Rubner), which states that fast
basal metabolic rate corresponds to short
maximum life span.
While there may be some validity to the idea that for various types
of specific damage detailed below that are by-products of metabolism,
all other things being equal, a fast metabolism may reduce lifespan, in
general this theory does not adequately explain the differences in
lifespan either within, or between, species. Calorically restricted
animals process as much, or more, calories per gram of body mass, as
their
ad libitum fed counterparts, yet exhibit substantially longer lifespans.
[citation needed] Similarly, metabolic rate is a poor predictor of lifespan for birds,
bats and other species that, it is presumed, have reduced mortality from
predation, and therefore have evolved long lifespans even in the
presence of very high metabolic rates.
[50]
In a 2007 analysis it was shown that, when modern statistical methods
for correcting for the effects of body size and phylogeny are employed,
metabolic rate does not correlate with longevity in mammals or birds.
[51] (For a critique of the
Rate of Living Hypothesis see
Living fast, dying when?[52])
With respect to specific types of chemical damage caused by metabolism, it is suggested that damage to long-lived
biopolymers, such as structural
proteins or
DNA, caused by ubiquitous chemical agents in the body such as
oxygen and
sugars, are in part responsible for aging. The damage can include breakage of biopolymer chains,
cross-linking of biopolymers, or chemical attachment of unnatural substituents (
haptens) to biopolymers.
Under normal
aerobic conditions, approximately 4% of the
oxygen metabolized by
mitochondria is converted to
superoxide ion, which can subsequently be converted to
hydrogen peroxide,
hydroxyl radical and eventually other reactive species including other
peroxides and
singlet oxygen, which can, in turn, generate
free radicals capable of damaging structural proteins and DNA. Certain metal
ions found in the body, such as
copper and
iron, may participate in the process. (In
Wilson's disease, a
hereditary defect that causes the body to retain copper, some of the symptoms resemble accelerated senescence.) These processes termed
oxidative stress are linked to the potential benefits of dietary
polyphenol antioxidants, for example in coffee,
[53] red wine and tea.
[54]
Sugars such as
glucose and
fructose can react with certain
amino acids such as
lysine and
arginine and certain DNA bases such as
guanine to produce sugar adducts, in a process called
glycation.
These adducts can further rearrange to form reactive species, which can
then cross-link the structural proteins or DNA to similar biopolymers
or other biomolecules such as non-structural proteins. People with
diabetes, who have elevated
blood sugar,
develop senescence-associated disorders much earlier than the general
population, but can delay such disorders by rigorous control of their
blood sugar levels. There is evidence that sugar damage is linked to
oxidant damage in a process termed
glycoxidation.
Free radicals can damage proteins,
lipids or DNA. Glycation mainly damages proteins. Damaged proteins and lipids accumulate in
lysosomes as
lipofuscin. Chemical damage to structural proteins can lead to loss of function; for example, damage to
collagen of
blood vessel walls can lead to vessel-wall stiffness and, thus,
hypertension, and vessel wall thickening and reactive tissue formation (
atherosclerosis); similar processes in the
kidney can lead to
renal failure. Damage to
enzymes reduces cellular functionality. Lipid
peroxidation of the inner mitochondrial membrane reduces the
electric potential and the ability to generate energy. It is probably no accident that nearly all of the so-called "
accelerated aging diseases" are due to defective
DNA repair enzymes.
It is believed that the
impact of alcohol on aging can be partly explained by alcohol's activation of the
HPA axis, which stimulates
glucocorticoid secretion, long-term exposure to which produces symptoms of aging.
[55]
DNA damage theory
Alexander
[56]
was the first to propose that DNA damage is the primary cause of aging.
Early experimental evidence supporting this idea was reviewed by
Gensler and Bernstein.
[57]
By the early 1990s experimental support for this proposal was
substantial, and further indicated that DNA damage due to reactive
oxygen species was a major source of the DNA damages causing aging.
[58][59][60][61][62] The current state of evidence bearing on this theory is reviewed in
DNA damage theory of aging and by Bernstein et al.
[63]
Reliability theory
Reliability theory
suggests that biological systems start their adult life with a high
load of initial damage. Reliability theory is a general theory about
systems failure. It allows researchers to predict the age-related
failure kinetics for a system of given architecture (
reliability structure)
and given reliability of its components. Reliability theory predicts
that even those systems which are composed entirely of non-aging
elements (with a constant
failure rate)
will nevertheless deteriorate (fail more often) with age, if these
systems are redundant in irreplaceable elements. Aging, therefore, is a
direct consequence of systems.
Reliability theory also predicts the
late-life mortality deceleration with subsequent leveling-off, as well as the late-life mortality plateaus, as an inevitable consequence of
redundancy exhaustion at extreme old ages. The theory explains why mortality rates increase exponentially with age (the
Gompertz law)
in many species, by taking into account the initial flaws (defects) in
newly formed systems. It also explains why organisms "prefer" to die
according to the Gompertz law, while technical devices usually fail
according to the
Weibull (power) law. Reliability theory allows to specify conditions when organisms die according to the
Weibull distribution:
Organisms should be relatively free of initial flaws and defects. The
theory makes it possible to find a general failure law applicable to all
adult and extreme old ages, where the Gompertz and the Weibull laws are
just special cases of this more general failure law. The theory
explains why relative differences in mortality rates of compared
populations (within a given species) vanish with age (
compensation law of mortality), and mortality convergence is observed due to the exhaustion of initial differences in redundancy levels.
Miscellanea
Biological clocks,
which objectively measure the biological age of cells and tissues, may
become useful for testing different biological aging theories.
[19]
A set of rare hereditary (
genetic) disorders, each called
progeria, has been known for some time. Sufferers exhibit symptoms resembling
accelerated aging, including wrinkled skin. The cause of
Hutchinson–Gilford progeria syndrome was reported in the journal
Nature in May 2003.
[64] This report suggests that
DNA damage, not
oxidative stress, is the cause of this form of accelerated aging.
Recently, a kind of early senescence has been alleged to be a possible unintended outcome of early
cloning experiments. The issue was raised in the case of
Dolly the sheep,
following her death from a contagious lung disease. The claim that
Dolly's early death involved premature senescence has been vigorously
contested,
[65] and Dolly's creator,
Dr. Ian Wilmut has expressed the view that her illness and death were probably unrelated to the fact that she was a clone.