From Wikipedia, the free encyclopedia
Molecular scale electronics, also called
single-molecule electronics, is a branch of
nanotechnology that uses single molecules, or nanoscale collections of single molecules, as
electronic components.
Because single molecules constitute the smallest stable structures
imaginable, this miniaturization is the ultimate goal for shrinking
electrical circuits.
The field is often termed simply as "
molecular electronics", but this term is also used to refer to the distantly related field of
conductive polymers and
organic electronics,
which uses the properties of molecules to affect the bulk properties of
a material. A nomenclature distinction has been suggested so that
molecular materials for electronics refers to this latter field of bulk applications, while
molecular scale electronics refers to the nanoscale single-molecule applications treated here.
[1][2]
Fundamental concepts
Conventional
electronics have traditionally been made from bulk materials. Ever
since their invention in 1958, the performance and complexity of
integrated circuits has undergone
exponential growth, a trend named
Moore’s law,
as feature sizes of the embedded components have shrunk accordingly. As
the structures shrink, the sensitivity to deviations increases. In a
few technology generations, when the minimum feature sizes reaches
13 nm, the composition of the devices must be controlled to a precision
of a few atoms
[3]
for the devices to work. With bulk methods growing increasingly
demanding and costly as they near inherent limits, the idea was born
that the components could instead be built up atom by atom in a
chemistry lab (bottom up) versus carving them out of bulk material (
top down).
This is the idea behind molecular electronics, with the ultimate
miniaturization being components contained in single molecules.
In single-molecule electronics, the bulk material is replaced by
single molecules. Instead of forming structures by removing or applying
material after a pattern scaffold, the atoms are put together in a
chemistry lab. In this way, billions of billions of copies are made
simultaneously (typically more than 10
20 molecules are made
at once) while the composition of molecules are controlled down to the
last atom. The molecules used have properties that resemble traditional
electronic components such as a
wire,
transistor or
rectifier.
Single-molecule electronics is an emerging field, and entire
electronic circuits consisting exclusively of molecular sized compounds
are still very far from being realized. However, the unceasing demand
for more computing power, along with the inherent limits of lithographic
methods as of 2016, make the transition seem unavoidable. Currently,
the focus is on discovering molecules with interesting properties and on
finding ways to obtain reliable and reproducible contacts between the
molecular components and the bulk material of the electrodes.
Theoretical basis
Molecular electronics operates in the
quantum realm of distances less than 100 nanometers. The miniaturization down to single molecules brings the scale down to a regime where
quantum mechanics effects are important. In conventional electronic components,
electrons can be filled in or drawn out more or less like a continuous flow of
electric charge.
In contrast, in molecular electronics the transfer of one electron
alters the system significantly. For example, when an electron has been
transferred from a source electrode to a molecule, the molecule gets
charged up, which makes it far harder for the next electron to transfer
(see also
Coulomb blockade).
The significant amount of energy due to charging must be accounted for
when making calculations about the electronic properties of the setup,
and is highly sensitive to distances to conducting surfaces nearby.
The theory of single-molecule devices is especially interesting since
the system under consideration is an open quantum system in
nonequilibrium
(driven by voltage). In the low bias voltage regime, the nonequilibrium
nature of the molecular junction can be ignored, and the
current-voltage traits of the device can be calculated using the
equilibrium electronic structure of the system. However, in stronger
bias regimes a more sophisticated treatment is required, as there is no
longer a
variational principle. In the elastic tunneling case (where the passing electron does not exchange energy with the system), the formalism of
Rolf Landauer
can be used to calculate the transmission through the system as a
function of bias voltage, and hence the current. In inelastic tunneling,
an elegant formalism based on the non-equilibrium
Green's functions of
Leo Kadanoff and
Gordon Baym, and independently by
Leonid Keldysh was advanced by
Ned Wingreen and
Yigal Meir.
This Meir-Wingreen formulation has been used to great success in the
molecular electronics community to examine the more difficult and
interesting cases where the transient electron exchanges energy with the
molecular system (for example through electron-phonon coupling or
electronic excitations).
Further, connecting single molecules reliably to a larger scale
circuit has proven a great challenge, and constitutes a significant
hindrance to commercialization.
Examples
Common
for molecules used in molecular electronics is that the structures
contain many alternating double and single bonds (see also
Conjugated system).
This is done because such patterns delocalize the molecular orbitals,
making it possible for electrons to move freely over the conjugated
area.
Wires
This animation of a rotating carbon nanotube shows its 3D structure.
The sole purpose of
molecular wires
is to electrically connect different parts of a molecular electrical
circuit. As the assembly of these and their connection to a macroscopic
circuit is still not mastered, the focus of research in single-molecule
electronics is primarily on the functionalized molecules: molecular
wires are characterized by containing no
functional groups and are hence composed of plain repetitions of a conjugated building block. Among these are the
carbon nanotubes that are quite large compared to the other suggestions but have shown very promising electrical properties.
The main problem with the molecular wires is to obtain good
electrical contact with the electrodes so that electrons can move freely
in and out of the wire.
Transistors
Single-molecule
transistors
are fundamentally different from the ones known from bulk electronics.
The gate in a conventional (field-effect) transistor determines the
conductance between the source and drain electrode by controlling the
density of charge carriers between them, whereas the gate in a
single-molecule transistor controls the possibility of a single electron
to jump on and off the molecule by modifying the energy of the
molecular orbitals. One of the effects of this difference is that the
single-molecule transistor is almost binary: it is either
on or
off. This opposes its bulk counterparts, which have quadratic responses to gate voltage.
It is the quantization of charge into electrons that is responsible
for the markedly different behavior compared to bulk electronics.
Because of the size of a single molecule, the charging due to a single
electron is significant and provides means to turn a transistor
on or
off (see
Coulomb blockade).
For this to work, the electronic orbitals on the transistor molecule
cannot be too well integrated with the orbitals on the electrodes. If
they are, an electron cannot be said to be located on the molecule or
the electrodes and the molecule will function as a wire.
A popular group of molecules, that can work as the
semiconducting
channel material in a molecular transistor, is the
oligopolyphenylenevinylenes (OPVs) that works by the Coulomb blockade
mechanism when placed between the source and drain electrode in an
appropriate way.
[4] Fullerenes work by the same mechanism and have also been commonly used.
Semiconducting carbon nanotubes have also been demonstrated to work
as channel material but although molecular, these molecules are
sufficiently large to behave almost as bulk
semiconductors.
The size of the molecules, and the low temperature of the
measurements being conducted, makes the quantum mechanical states well
defined. Thus, it is being researched if the quantum mechanical
properties can be used for more advanced purposes than simple
transistors (e.g.
spintronics).
Physicists at the
University of Arizona, in collaboration with chemists from the
University of Madrid, have designed a single-molecule transistor using a ring-shaped molecule similar to
benzene. Physicists at Canada's
National Institute for Nanotechnology
have designed a single-molecule transistor using styrene. Both groups
expect (the designs were experimentally unverified as of June 2005) their respective devices to function at room temperature, and to be controlled by a single electron.
[5]
Rectifiers (diodes)
Hydrogen can be removed from individual
tetraphenylporphyrin (H
2TPP) molecules by applying excess voltage to the tip of a
scanning tunneling microscope (STAM, a); this removal alters the current-voltage (I-V) curves of TPP molecules, measured using the same STM tip, from
diode-like (red curve in b) to
resistor-like (green curve). Image (c) shows a row of TPP, H
2TPP and TPP molecules. While scanning image (d), excess voltage was applied to H
2TPP
at the black dot, which instantly removed hydrogen, as shown in the
bottom part of (d) and in the re-scan image (e). Such manipulations can
be used in single-molecule electronics.
[6]
Molecular
rectifiers
are mimics of their bulk counterparts and have an asymmetric
construction so that the molecule can accept electrons in one end but
not the other. The molecules have an
electron donor (D) in one end and an
electron acceptor (A) in the other. This way, the unstable state D
+ – A
− will be more readily made than D
− – A
+. The result is that an
electric current
can be drawn through the molecule if the electrons are added through
the acceptor end, but less easily if the reverse is attempted.
Methods
One of
the biggest problems with measuring on single molecules is to establish
reproducible electrical contact with only one molecule and doing so
without shortcutting the electrodes. Because the current
photolithographic
technology is unable to produce electrode gaps small enough to contact
both ends of the molecules tested (on the order of nanometers),
alternative strategies are applied.
Molecular gaps
One
way to produce electrodes with a molecular sized gap between them is
break junctions, in which a thin electrode is stretched until it breaks.
Another is
electromigration.
Here a current is led through a thin wire until it melts and the atoms
migrate to produce the gap. Further, the reach of conventional
photolithography can be enhanced by chemically etching or depositing
metal on the electrodes.
Probably the easiest way to conduct measurements on several molecules is to use the tip of a
scanning tunneling microscope (STM) to contact molecules adhered at the other end to a metal substrate.
[7]
Anchoring
A popular way to anchor molecules to the electrodes is to make use of
sulfur's high
chemical affinity to
gold. In these setups, the molecules are
synthesized so that sulfur atoms are placed strategically to function as
crocodile clips
connecting the molecules to the gold electrodes. Though useful, the
anchoring is non-specific and thus anchors the molecules randomly to all
gold surfaces. Further, the
contact resistance
is highly dependent on the precise atomic geometry around the site of
anchoring and thereby inherently compromises the reproducibility of the
connection.
To circumvent the latter issue, experiments has shown that
fullerenes
could be a good candidate for use instead of sulfur because of the
large conjugated π-system that can electrically contact many more atoms
at once than one atom of sulfur.
[8]
Fullerene nanoelectronics
In
polymers,
classical organic molecules are composed of both carbon and hydrogen
(and sometimes additional compounds such as nitrogen, chlorine or
sulphur). They are obtained from petrol and can often be synthesized in
large amounts. Most of these molecules are insulating when their length
exceeds a few nanometers. However, naturally occurring carbon is
conducting, especially graphite recovered from coal or encountered
otherwise. From a theoretical viewpoint,
graphite is a
semi-metal,
a category in between metals and semi-conductors. It has a layered
structure, each sheet being one atom thick. Between each sheet, the
interactions are weak enough to allow an easy manual cleavage.
Tailoring the
graphite
sheet to obtain well defined nanometer-sized objects remains a
challenge. However, by the close of the twentieth century, chemists were
exploring methods to fabricate extremely small graphitic objects that
could be considered single molecules. After studying the interstellar
conditions under which carbon is known to form clusters,
Richard Smalley's
group (Rice University, Texas) set up an experiment in which graphite
was vaporized via laser irradiation. Mass spectrometry revealed that
clusters containing specific
magic numbers of atoms were stable, especially those clusters of 60 atoms.
Harry Kroto,
an English chemist who assisted in the experiment, suggested a possible
geometry for these clusters – atoms covalently bound with the exact
symmetry of a soccer ball. Coined
buckminsterfullerenes, buckyballs, or C
60,
the clusters retained some properties of graphite, such as
conductivity. These objects were rapidly envisioned as possible building
blocks for molecular electronics.
Problems
Artifacts
When
trying to measure electronic traits of molecules, artificial phenomena
can occur that can be hard to distinguish from truly molecular behavior.
[9]
Before they were discovered, these artifacts have mistakenly been
published as being features pertaining to the molecules in question.
Applying a voltage drop on the order of volts across a nanometer
sized junction results in a very strong electrical field. The field can
cause metal atoms to migrate and eventually close the gap by a thin
filament, which can be broken again when carrying a current. The two
levels of conductance imitate molecular switching between a conductive
and an isolating state of a molecule.
Another encountered artifact is when the electrodes undergo chemical
reactions due to the high field strength in the gap. When the
voltage bias is reversed, the reaction will cause
hysteresis in the measurements that can be interpreted as being of molecular origin.
A metallic grain between the electrodes can act as a single electron
transistor by the mechanism described above, thus resembling the traits
of a molecular transistor. This artifact is especially common with
nanogaps produced by the electromigration method.
Commercialization
One
of the biggest hindrances for single-molecule electronics to be
commercially exploited is the lack of methods to connect a molecular
sized circuit to bulk electrodes in a way that gives reproducible
results. At the current state, the difficulty of connecting single
molecules vastly outweighs any possible performance increase that could
be gained from such shrinkage. The difficulties grow worse if the
molecules are to have a certain spatial orientation and/or have multiple
poles to connect.
Also problematic is that some measurements on single molecules are carried out in
cryogenic temperatures (near absolute zero), which is very energy consuming. This is done to reduce
signal noise enough to measure the faint currents of single molecules.
History and recent progress
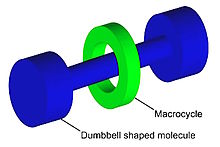
Graphical representation of a
rotaxane, useful as a molecular switch.
In their treatment of so-called
donor-acceptor complexes in the 1940s,
Robert Mulliken and
Albert Szent-Györgyi
advanced the concept of charge transfer in molecules. They subsequently
further refined the study of both charge transfer and energy transfer
in molecules. Likewise, a 1974 paper from
Mark Ratner and Ari Aviram illustrated a theoretical molecular
rectifier.
[10] In 1988, Aviram described in detail a theoretical single-molecule
field-effect transistor. Further concepts were proposed by Forrest Carter of the
Naval Research Laboratory, including single-molecule
logic gates. A wide range of ideas were presented, under his aegis, at a conference entitled
Molecular Electronic Devices in 1988.
[11] These were all theoretical constructs and not concrete devices. The
direct
measurement of the electronic traits of individual molecules awaited
the development of methods for making molecular-scale electrical
contacts. This was no easy task. Thus, the first experiment
directly-measuring the conductance of a single molecule was only
reported in 1995 on a single C
60 molecule by C. Joachim and
J. K. Gimzewsky in their seminal Physical Revie Letter paper and later
in 1997 by Mark Reed and co-workers on a few hundred molecules. Since
then, this branch of the field has advanced rapidly. Likewise, as it has
grown possible to measure such properties directly, the theoretical
predictions of the early workers have been confirmed substantially.
Recent progress in
nanotechnology and nanoscience has facilitated both experimental and theoretical study of molecular electronics. Development of the
scanning tunneling microscope (STM) and later the
atomic force microscope
(AFM) have greatly facilitated manipulating single-molecule
electronics. Also, theoretical advances in molecular electronics have
facilitated further understanding of non-adiabatic charge transfer
events at electrode-electrolyte interfaces.
[12][13]
The concept of molecular electronics was first published in 1974 when
Aviram and Ratner suggested an organic molecule that could work as a
rectifier.
[14]
Having both huge commercial and fundamental interest, much effort was
put into proving its feasibility, and 16 years later in 1990, the first
demonstration of an intrinsic molecular rectifier was realized by
Ashwell and coworkers for a thin film of molecules.
The first measurement of the conductance of a single molecule was
realised in 1994 by C. Joachim and J. K. Gimzewski and published in 1995
(see the corresponding Phys. Rev. Lett. paper). This was the conclusion
of 10 years of research started at IBM TJ Watson, using the scanning
tunnelling microscope tip apex to switch a single molecule as already
explored by A. Aviram, C. Joachim and M. Pomerantz at the end of the
80's (see their seminal Chem. Phys. Lett. paper during this period). The
trick was to use an UHV Scanning Tunneling microscope to allow the tip
apex to gently touch the top of a single
C
60
molecule adsorbed on an Au(110) surface. A resistance of 55 MOhms was
recorded along with a low voltage linear I-V. The contact was certified
by recording the I-z current distance property, which allows measurement
of the deformation of the
C
60
cage under contact. This first experiment was followed by the reported
result using a mechanical break junction method to connect two gold
electrodes to a sulfur-terminated
molecular wire by
Mark Reed and
James Tour in 1997.
[15]
A single-molecule amplifier was implemented by C. Joachim and J.K. Gimzewski in IBM Zurich. This experiment, involving one
C
60
molecule, demonstrated that one such molecule can provide gain in a
circuit via intramolecular quantum interference effects alone.
A collaboration of researchers at
Hewlett-Packard (HP) and
University of California, Los Angeles
(UCLA), led by James Heath, Fraser Stoddart, R. Stanley Williams, and
Philip Kuekes, has developed molecular electronics based on
rotaxanes and
catenanes.
Work is also occurring on the use of single-wall carbon nanotubes as
field-effect transistors. Most of this work is being done by
International Business Machines (
IBM).
Some specific reports of a
field-effect transistor based on molecular
self-assembled monolayers were shown to be fraudulent in 2002 as part of the
Schön scandal.
[16]
Until recently entirely theoretical, the Aviram-Ratner model for a
unimolecular rectifier has been confirmed unambiguously in experiments by a group led by Geoffrey J. Ashwell at
Bangor University, UK.
[17][18][19] Many rectifying molecules have so far been identified, and the number and efficiency of these systems is growing rapidly.
Supramolecular electronics is a new field involving electronics at a
supramolecular level.
An important issue in molecular electronics is the determination of
the resistance of a single molecule (both theoretical and experimental).
For example, Bumm, et al. used STM to analyze a single molecular switch
in a
self-assembled monolayer to determine how conductive such a molecule can be.
[20]
Another problem faced by this field is the difficulty of performing
direct characterization since imaging at the molecular scale is often
difficult in many experimental devices.