This
diagram of the fast carbon cycle shows the movement of carbon between
land, atmosphere, soil and oceans in billions of tons of carbon per
year. Yellow numbers are natural fluxes, red are human contributions in
billions of tons of carbon per year. White numbers indicate stored
carbon.
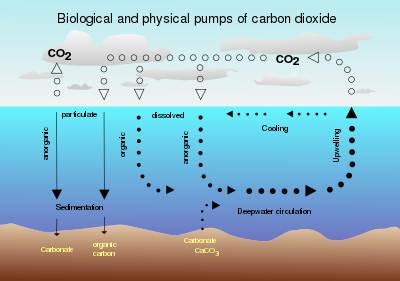
Air-sea exchange of CO2
A carbon sink is a natural or artificial reservoir that accumulates and stores some carbon-containing chemical compound for an indefinite period. The process by which carbon sinks remove carbon dioxide (CO2) from the atmosphere is known as carbon sequestration. Public awareness of the significance of CO2 sinks has grown since passage of the Kyoto Protocol, which promotes their use as a form of carbon offset. There are also different strategies used to enhance this process.
General
Increase in atmospheric carbon dioxide means increase in global temperature. The amount of carbon dioxide varies naturally. The natural sinks are:
- Trees serve as carbon sinks during growing seasons.
- Absorption of carbon dioxide by the oceans via physicochemical and biological processes
- Photosynthesis by terrestrial plants
Whilst the creation of artificial sinks has been discussed, no major artificial systems remove carbon from the atmosphere on a material scale.
Carbon sources include:
- Combustion of fossil fuels (coal, natural gas, and oil) by humans for energy and transportation
- Farmland (by animal respiration); there are proposals for improvements in farming practices to reverse this.
Kyoto Protocol
Because growing vegetation takes in carbon dioxide, the Kyoto Protocol allows Annex I countries with large areas of growing forests to issue Removal Units
to recognize the sequestration of carbon. The additional units make it
easier for them to achieve their target emission levels. It is estimated
that forests absorb between 10 and 20 tons of carbon dioxide per hectare each year, through photosynthetic conversion into starch, cellulose, lignin, and wooden biomass. While this has been well documented for temperate forests and plantations, the fauna of the tropical forests place some limitations for such global estimates.
Some countries seek to trade emission rights in carbon emission
markets, purchasing the unused carbon emission allowances of other
countries. If overall limits on greenhouse gas emission are put into
place, cap and trade market mechanisms are purported to find cost-effective ways to reduce emissions. There is as yet no carbon audit regime for all such markets globally, and none is specified in the Kyoto Protocol. National carbon emissions are self-declared.
In the Clean Development Mechanism, only afforestation and reforestation are eligible to produce certified emission reductions (CERs) in the first commitment period of the Kyoto Protocol (2008–2012). Forest conservation activities or activities avoiding deforestation, which would result in emission reduction through the conservation of existing carbon stocks, are not eligible at this time. Also, agricultural carbon sequestration is not possible yet.
Storage in terrestrial and marine environments
Soils
Soils
represent a short to long-term carbon storage medium, and contain more
carbon than all terrestrial vegetation and the atmosphere combined. Plant litter and other biomass including charcoal accumulates as organic matter in soils, and is degraded by chemical weathering and biological degradation. More recalcitrant organic carbon polymers such as cellulose, hemi-cellulose, lignin, aliphatic compounds, waxes and terpenoids are collectively retained as humus. Organic matter tends to accumulate in litter and soils of colder regions such as the boreal forests of North America and the Taiga of Russia. Leaf litter and humus are rapidly oxidized and poorly retained in sub-tropical and tropical climate conditions due to high temperatures and extensive leaching by rainfall. Areas where shifting cultivation or slash and burn
agriculture are practiced are generally only fertile for 2–3 years
before they are abandoned. These tropical jungles are similar to coral
reefs in that they are highly efficient at conserving and circulating
necessary nutrients, which explains their lushness in a nutrient desert. Much organic carbon retained in many agricultural areas worldwide has been severely depleted due to intensive farming practices.
Grasslands contribute to soil organic matter,
stored mainly in their extensive fibrous root mats. Due in part to the
climatic conditions of these regions (e.g. cooler temperatures and
semi-arid to arid conditions), these soils can accumulate significant
quantities of organic matter. This can vary based on rainfall, the
length of the winter season, and the frequency of naturally occurring
lightning-induced grass-fires.
While these fires release carbon dioxide, they improve the quality of
the grasslands overall, in turn increasing the amount of carbon retained
in the humic material. They also deposit carbon directly to the soil
in the form of char that does not significantly degrade back to carbon dioxide.
Forest fires release absorbed carbon back into the atmosphere, as does deforestation due to rapidly increased oxidation of soil organic matter.
Organic matter in peat bogs undergoes slow anaerobic decomposition below the surface. This process is slow enough that in many cases the bog grows rapidly and fixes
more carbon from the atmosphere than is released. Over time, the peat
grows deeper. Peat bogs hold approximately one-quarter of the carbon
stored in land plants and soils.
Under some conditions, forests and peat bogs may become sources of CO2,
such as when a forest is flooded by the construction of a hydroelectric
dam. Unless the forests and peat are harvested before flooding, the
rotting vegetation is a source of CO2 and methane comparable in magnitude to the amount of carbon released by a fossil-fuel powered plant of equivalent power.
Regenerative agriculture
Current agricultural practices lead to carbon loss from soils. It has
been suggested that improved farming practices could return the soils
to being a carbon sink. Present worldwide practices of overgrazing are
substantially reducing many grasslands' performance as carbon sinks. The Rodale Institute says that regenerative agriculture, if practiced on the planet’s 3.6 billion tillable acres, could sequester up to 40% of current CO2 emissions. They claim that agricultural carbon sequestration has the potential to mitigate global warming. When using biologically based regenerative practices, this dramatic benefit can be accomplished with no decrease in yields or farmer profits. Organically managed soils can convert carbon dioxide from a greenhouse gas into a food-producing asset (DJS: As organically grown food requires more land I dispute this claim.).
In 2006, U.S. carbon dioxide emissions, largely from fossil fuel combustion, were estimated at nearly 6.5 billion tons. If a 2,000 (lb/ac)/year sequestration rate was achieved on all 434,000,000 acres (1,760,000 km2)
of cropland in the United States, nearly 1.6 billion tons of carbon
dioxide would be sequestered per year, mitigating close to one quarter
of the country's total fossil fuel emissions.
Oceans
Presently, oceans are CO2
sinks, and represent the largest active carbon sink on Earth, absorbing
more than a quarter of the carbon dioxide that humans put into the air. The solubility pump is the primary mechanism responsible for the CO2 absorption by the oceans.
The biological pump plays a negligible role, because of the limitation to pump by ambient light and nutrients required by the phytoplankton that ultimately drive it. Total inorganic carbon is not believed to limit primary production
in the oceans, so its increasing availability in the ocean does not
directly affect production (the situation on land is different, since
enhanced atmospheric levels of CO2 essentially "fertilize" land plant growth to some threshold). However, ocean acidification by invading anthropogenic CO2 may affect the biological pump by negatively impacting calcifying organisms such as coccolithophores, foraminiferans and pteropods. Climate change may also affect the biological pump in the future by warming and stratifying the surface ocean, thus reducing the supply of limiting nutrients to surface waters.
A 2008 study found that CO2 could potentially increase primary productivity, particularly in eel grasses in coastal and estuarine habitats.
In January 2009, the Monterey Bay Aquarium Research Institute and the National Oceanic and Atmospheric Administration
announced a joint study to determine whether the ocean off the
California coast was serving as a carbon source or a carbon sink.
Principal instrumentation for the study will be self-contained CO2 monitors placed on buoys in the ocean. They will measure the partial pressure of CO2 in the ocean and the atmosphere just above the water surface.
In February 2009, Science Daily reported that the Southern Indian
Ocean is becoming less effective at absorbing carbon dioxide due to
changes to the region's climate which include higher wind speeds.
On longer timescales, oceans may be both sources and sinks – during ice ages CO2 levels decrease to ≈180 ppmv, and much of this is believed to be stored in the oceans. As ice ages end, CO2 is released from the oceans and CO2 levels during previous interglacials have been around ≈280 ppmv. This role as a sink for CO2 is driven by two processes, the solubility pump and the biological pump. The former is primarily a function of differential CO2 solubility in seawater and the thermohaline circulation, while the latter is the sum of a series of biological processes that transport carbon (in organic and inorganic forms) from the surface euphotic zone to the ocean's interior. A small fraction of the organic carbon transported by the biological pump to the seafloor is buried in anoxic conditions under sediments and ultimately forms fossil fuels such as oil and natural gas.
At the end of glacials with sea level rapidly rising, corals tend
to grow slower due to increased ocean temperature as seen on the
Showtime series "Years of Living Dangerously". The calcium carbonate
from which coral skeletons are made is just over 60% carbon dioxide. If
we postulate that coral reefs were eroded down to the glacial sea
level, then coral reefs have grown 120m upward since the end of the
recent glacial.
Enhancing natural sequestration
Forests
Forests can be carbon stores, and they are carbon dioxide sinks when they are increasing in density or area. In Canada's boreal forests as much as 80% of the total carbon is stored in the soils as dead organic matter. A 40-year study of African, Asian, and South American tropical forests
by the University of Leeds, shows tropical forests absorb about 18% of
all carbon dioxide added by fossil fuels. Truly mature tropical forests,
by definition, grow rapidly as each tree produces at least 10 new trees
each year. Based on studies of the FAO and UNEP
it has been estimated that Asian forests absorb about 5 tonnes of
carbon dioxide per hectare each year. The global cooling effect of
carbon sequestration by forests is partially counterbalanced in that
reforestation can decrease the reflection of sunlight (albedo). Mid-to-high latitude forests have a much lower albedo
during snow seasons than flat ground, thus contributing to warming.
Modeling that compares the effects of albedo differences between forests
and grasslands suggests that expanding the land area of forests in
temperate zones offers only a temporary cooling benefit.
In the United States in 2004 (the most recent year for which EPA statistics are available), forests sequestered 10.6% (637 MegaTonnes)
of the carbon dioxide released in the United States by the combustion
of fossil fuels (coal, oil and natural gas; 5657 MegaTonnes). Urban trees sequestered another 1.5% (88 MegaTonnes). To further reduce U.S. carbon dioxide emissions by 7%, as stipulated by the Kyoto Protocol, would require the planting of "an area the size of Texas [8% of the area of Brazil] every 30 years". Carbon offset
programs are planting millions of fast-growing trees per year to
reforest tropical lands, for as little as $0.10 per tree; over their
typical 40-year lifetime, one million of these trees will fix 1 to 2
MegaTonnes of carbon dioxide.
In Canada, reducing timber harvesting would have very little impact on
carbon dioxide emissions because of the combination of harvest and
stored carbon in manufactured wood products along with the regrowth of
the harvested forests. Additionally, the amount of carbon released from
harvesting is small compared to the amount of carbon lost each year to
forest fires and other natural disturbances.
The Intergovernmental Panel on Climate Change
concluded that "a sustainable forest management strategy aimed at
maintaining or increasing forest carbon stocks, while producing an
annual sustained yield of timber fiber or energy from the forest, will
generate the largest sustained mitigation benefit".
Sustainable management practices keep forests growing at a higher rate
over a potentially longer period of time, thus providing net
sequestration benefits in addition to those of unmanaged forests.
Life expectancy of forests varies throughout the world,
influenced by tree species, site conditions and natural disturbance
patterns. In some forests carbon may be stored for centuries, while in
other forests carbon is released with frequent stand replacing fires.
Forests that are harvested prior to stand replacing events allow for the
retention of carbon in manufactured forest products such as lumber.
However, only a portion of the carbon removed from logged forests ends
up as durable goods and buildings. The remainder ends up as sawmill
by-products such as pulp, paper and pallets, which often end with
incineration (resulting in carbon release into the atmosphere) at the
end of their lifecycle. For instance, of the 1,692 MegaTonnes of carbon
harvested from forests in Oregon and Washington (U.S) from 1900 to 1992,
only 23% is in long-term storage in forest products.
Oceans
One way to increase the carbon sequestration efficiency of the oceans
is to add micrometre-sized iron particles in the form of either hematite (iron oxide) or melanterite (iron sulfate) to certain regions of the ocean. This has the effect of stimulating growth of plankton. Iron is an important nutrient for phytoplankton, usually made available via upwelling along the continental shelves, inflows from rivers and streams, as well as deposition of dust suspended in the atmosphere.
Natural sources of ocean iron have been declining in recent decades,
contributing to an overall decline in ocean productivity (NASA, 2003). Yet in the presence of iron nutrients plankton populations quickly grow, or 'bloom', expanding the base of biomass productivity throughout the region and removing significant quantities of CO2 from the atmosphere via photosynthesis. A test in 2002 in the Southern Ocean around Antarctica suggests that between 10,000 and 100,000 carbon atoms are sunk for each iron atom added to the water.
More recent work in Germany (2005) suggests that any biomass carbon in
the oceans, whether exported to depth or recycled in the euphotic zone,
represents long-term storage of carbon. This means that application of
iron nutrients in select parts of the oceans, at appropriate scales,
could have the combined effect of restoring ocean productivity while at
the same time mitigating the effects of human caused emissions of carbon
dioxide to the atmosphere.
Because the effect of periodic small scale phytoplankton blooms
on ocean ecosystems is unclear, more studies would be helpful.
Phytoplankton have a complex effect on cloud formation via the release
of substances such as dimethyl sulfide (DMS) that are converted to sulfate aerosols in the atmosphere, providing cloud condensation nuclei, or CCN. But the effect of small scale plankton blooms on overall DMS production is unknown.
Other nutrients such as nitrates, phosphates, and silica as well
as iron may cause ocean fertilization. There has been some speculation
that using pulses of fertilization (around 20 days in length) may be
more effective at getting carbon to ocean floor than sustained
fertilization.
There is some controversy over seeding the oceans with iron
however, due to the potential for increased toxic phytoplankton growth
(e.g. "red tide"),
declining water quality due to overgrowth, and increasing anoxia in
areas harming other sea-life such as zooplankton, fish, coral, etc.
Soils
Since the
1850s, a large proportion of the world's grasslands have been tilled
and converted to croplands, allowing the rapid oxidation of large
quantities of soil organic carbon. However, in the United States in
2004 (the most recent year for which EPA statistics are available),
agricultural soils including pasture land sequestered 0.8% (46 teragrams) as much carbon as was released in the United States by the combustion of fossil fuels (5988 teragrams). The annual amount of this sequestration has been gradually increasing since 1998.
Methods that significantly enhance carbon sequestration in soil include no-till farming, residue mulching, cover cropping, and crop rotation, all of which are more widely used in organic farming than in conventional farming.
Because only 5% of US farmland currently uses no-till and residue
mulching, there is a large potential for carbon sequestration. Conversion to pastureland, particularly with good management of grazing, can sequester even more carbon in the soil.
Terra preta, an anthropogenic, high-carbon soil, is also being investigated as a sequestration mechanism.
By pyrolysing biomass, about half of its carbon can be reduced to charcoal, which can persist in the soil for centuries, and makes a useful soil amendment, especially in tropical soils (biochar or agrichar).
Savanna
Controlled burns on far north Australian savannas
can result in an overall carbon sink. One working example is the West
Arnhem Fire Management Agreement, started to bring "strategic fire
management across 28,000 km² of Western Arnhem Land". Deliberately
starting controlled burns early in the dry season results in a mosaic of
burnt and unburnt country which reduces the area of burning compared
with stronger, late dry season fires. In the early dry season there are
higher moisture levels, cooler temperatures, and lighter wind than later
in the dry season; fires tend to go out overnight. Early controlled
burns also results in a smaller proportion of the grass and tree biomass
being burnt. Emission reductions of 256,000 tonnes of CO2 have been made as of 2007.
Artificial sequestration
For carbon to be sequestered artificially (i.e. not using the natural processes of the carbon cycle) it must first be captured, or
it must be significantly delayed or prevented from being re-released
into the atmosphere (by combustion, decay, etc.) from an existing
carbon-rich material, by being incorporated into an enduring usage (such
as in construction). Thereafter it can be passively stored or remain productively utilized over time in a variety of ways.
For example, upon harvesting, wood (as a carbon-rich material)
can be immediately burned or otherwise serve as a fuel, returning its
carbon to the atmosphere, or it can be incorporated into
construction or a range of other durable products, thus sequestering its
carbon over years or even centuries.
Indeed, a very carefully designed and durable, energy-efficient
and energy-capturing building has the potential to sequester (in its
carbon-rich construction materials), as much as or more carbon than was
released by the acquisition and incorporation of all its materials and
than will be released by building-function "energy-imports" during the
structure's (potentially multi-century) existence. Such a structure
might be termed "carbon neutral" or even "carbon negative". Building
construction and operation (electricity usage, heating, etc.) are
estimated to contribute nearly half of the annual human-caused carbon additions to the atmosphere.
Natural-gas purification plants often already have to remove carbon dioxide, either to avoid dry ice
clogging gas tankers or to prevent carbon-dioxide concentrations
exceeding the 3% maximum permitted on the natural-gas distribution grid.
Beyond this, one of the most likely early applications of carbon capture is the capture of carbon dioxide from flue gases at power stations (in the case of coal, this coal pollution mitigation is sometimes known as "clean coal"). A typical new 1000 MW coal-fired power station
produces around 6 million tons of carbon dioxide annually. Adding
carbon capture to existing plants can add significantly to the costs of
energy production; scrubbing costs aside, a 1000 MW coal plant will
require the storage of about 50 million barrels (7,900,000 m3) of carbon dioxide a year. However, scrubbing is relatively affordable when added to new plants based on coal gasification
technology, where it is estimated to raise energy costs for households
in the United States using only coal-fired electricity sources from 10
cents per kW·h to 12 cents.
Carbon capture
Currently, capture of carbon dioxide is performed on a large scale by absorption of carbon dioxide onto various amine-based solvents. Other techniques are currently being investigated, such as pressure swing adsorption, temperature swing adsorption, gas separation membranes, cryogenics and flue capture.
In coal-fired power stations, the main alternatives to
retrofitting amine-based absorbers to existing power stations are two
new technologies: coal gasification combined-cycle and oxy-fuel combustion. Gasification first produces a "syngas" primarily of hydrogen and carbon monoxide, which is burned, with carbon dioxide filtered from the flue gas. Oxy-fuel combustion burns the coal in oxygen instead of air, producing only carbon dioxide and water vapour,
which are relatively easily separated. Some of the combustion products
must be returned to the combustion chamber, either before or after
separation, otherwise the temperatures would be too high for the
turbine.
Another long-term option is carbon capture directly from the air using hydroxides. The air would literally be scrubbed of its CO2 content. This idea offers an alternative to non-carbon-based fuels for the transportation sector.
Examples of carbon sequestration at coal plants include converting carbon from smokestacks into baking soda, and algae-based carbon capture, circumventing storage by converting algae into fuel or feed.
Oceans
Another
proposed form of carbon sequestration in the ocean is direct injection.
In this method, carbon dioxide is pumped directly into the water at
depth, and expected to form "lakes" of liquid CO2 at the bottom. Experiments carried out in moderate to deep waters (350–3600 m) indicate that the liquid CO2 reacts to form solid CO2 clathrate hydrates, which gradually dissolve in the surrounding waters.
This method, too, has potentially dangerous environmental consequences. The carbon dioxide does react with the water to form carbonic acid, H2CO3; however, most (as much as 99%) remains as dissolved molecular CO2.
The equilibrium would no doubt be quite different under the high
pressure conditions in the deep ocean. In addition, if deep-sea
bacterial methanogens that reduce carbon dioxide were to encounter the carbon dioxide sinks, levels of methane gas may increase, leading to the generation of an even worse greenhouse gas.
The resulting environmental effects on benthic life forms of the bathypelagic, abyssopelagic and hadopelagic
zones are unknown. Even though life appears to be rather sparse in the
deep ocean basins, energy and chemical effects in these deep basins
could have far-reaching implications. Much more work is needed here to
define the extent of the potential problems.
Carbon storage in or under oceans may not be compatible with the Convention on the Prevention of Marine Pollution by Dumping of Wastes and Other Matter.
An additional method of long-term ocean-based sequestration is to gather crop residue such as corn stalks or excess hay into large weighted bales of biomass and deposit it in the alluvial fan areas of the deep ocean basin.
Dropping these residues in alluvial fans would cause the residues to
be quickly buried in silt on the sea floor, sequestering the biomass for
very long time spans. Alluvial fans exist in all of the world's oceans
and seas where river deltas fall off the edge of the continental shelf such as the Mississippi alluvial fan in the gulf of Mexico and the Nile alluvial fan in the Mediterranean Sea.
A downside, however, would be an increase in aerobic bacteria growth
due to the introduction of biomass, leading to more competition for
oxygen resources in the deep sea, similar to the oxygen minimum zone.
Geological sequestration
The method of geo-sequestration or geological storage involves injecting carbon dioxide directly into underground geological formations. Declining oil fields, saline aquifers, and unminable coal seams
have been suggested as storage sites. Caverns and old mines that are
commonly used to store natural gas are not considered, because of a lack
of storage safety.
CO2 has been injected into declining oil fields for
more than 40 years, to increase oil recovery. This option is attractive
because the storage costs are offset by the sale of additional oil that
is recovered. Typically, 10–15% additional recovery of the original oil
in place is possible. Further benefits are the existing infrastructure
and the geophysical and geological information about the oil field that
is available from the oil exploration. Another benefit of injecting CO2 into Oil fields is that CO2 is soluble in oil. Dissolving CO2
in oil lowers the viscosity of the oil and reduces its interfacial
tension which increases the oils mobility. All oil fields have a
geological barrier preventing upward migration of oil. As most oil and
gas has been in place for millions to tens of millions of years,
depleted oil and gas reservoirs can contain carbon dioxide for
millennia. Identified possible problems are the many 'leak'
opportunities provided by old oil wells, the need for high injection
pressures and acidification which can damage the geological barrier.
Other disadvantages of old oil fields are their limited geographic
distribution and depths, which require high injection pressures for
sequestration. Below a depth of about 1000 m, carbon dioxide is
injected as a supercritical fluid, a material with the density of a
liquid, but the viscosity and diffusivity of a gas.
Unminable coal seams can be used to store CO2, because CO2
absorbs to the coal surface, ensuring safe long-term storage. In the
process it releases methane that was previously adsorbed to the coal
surface and that may be recovered. Again the sale of the methane can be
used to offset the cost of the CO2 storage. Release or
burning of methane would of course at least partially offset the
obtained sequestration result – except when the gas is allowed to escape
into the atmosphere in significant quantities: methane has a higher global warming potential than CO2.
Saline aquifers contain highly mineralized brines and have so far
been considered of no benefit to humans except in a few cases where
they have been used for the storage of chemical waste. Their advantages
include a large potential storage volume and relatively common
occurrence reducing the distance over which CO2 has to be
transported. The major disadvantage of saline aquifers is that
relatively little is known about them compared to oil fields. Another
disadvantage of saline aquifers is that as the salinity of the water
increases, less CO2 can be dissolved into aqueous solution.
To keep the cost of storage acceptable the geophysical exploration may
be limited, resulting in larger uncertainty about the structure of a
given aquifer. Unlike storage in oil fields or coal beds, no side
product will offset the storage cost. Leakage of CO2 back into the atmosphere may be a problem in saline-aquifer storage. However, current research shows that several trapping mechanisms immobilize the CO2 underground, reducing the risk of leakage.
A major research project examining the geological sequestration
of carbon dioxide is currently being performed at an oil field at Weyburn in south-eastern Saskatchewan. In the North Sea, Norway's Equinor natural-gas platform Sleipner
strips carbon dioxide out of the natural gas with amine solvents and
disposes of this carbon dioxide by geological sequestration. Sleipner
reduces emissions of carbon dioxide by approximately one million tonnes a
year. The cost of geological sequestration is minor relative to the
overall running costs. As of April 2005, BP is considering a trial of large-scale sequestration of carbon dioxide stripped from power plant emissions in the Miller oilfield as its reserves are depleted.
In October 2007, the Bureau of Economic Geology at The University of Texas at Austin
received a 10-year, $38 million subcontract to conduct the first
intensively monitored, long-term project in the United States studying
the feasibility of injecting a large volume of CO2 for underground storage. The project is a research program of the Southeast Regional Carbon Sequestration Partnership (SECARB), funded by the National Energy Technology Laboratory of the U.S. Department of Energy (DOE). The SECARB partnership will demonstrate CO2 injection rate and storage capacity in the Tuscaloosa-Woodbine geologic system that stretches from Texas to Florida. Beginning in fall 2007, the project will inject CO2 at the rate of one million tons per year, for up to 1.5 years, into brine up to 10,000 feet (3,000 m) below the land surface near the Cranfield oil field about 15 miles (24 km) east of Natchez, Mississippi. Experimental equipment will measure the ability of the subsurface to accept and retain CO2.
Mineral sequestration
Mineral sequestration aims to trap carbon in the form of solid carbonate salts. This process occurs slowly in nature and is responsible for the deposition and accumulation of limestone over geologic time. Carbonic acid in groundwater slowly reacts with complex silicates to dissolve calcium, magnesium, alkalis and silica and leave a residue of clay minerals. The dissolved calcium and magnesium react with bicarbonate
to precipitate calcium and magnesium carbonates, a process that
organisms use to make shells. When the organisms die, their shells are
deposited as sediment and eventually turn into limestone. Limestones
have accumulated over billions of years of geologic time and contain
much of Earth's carbon. Ongoing research aims to speed up similar
reactions involving alkali carbonates.
Several serpentinite deposits are being investigated as potentially large scale CO2 storage sinks such as those found in NSW, Australia, where the first mineral carbonation pilot plant project is underway.
Beneficial re-use of magnesium carbonate from this process could
provide feedstock for new products developed for the built environment
and agriculture without returning the carbon into the atmosphere and so
acting as a carbon sink.
One proposed reaction is that of the olivine-rich rock dunite, or its hydrated equivalent serpentinite with carbon dioxide to form the carbonate mineral magnesite, plus silica and iron oxide (magnetite).
Serpentinite sequestration is favored because of the non-toxic
and stable nature of magnesium carbonate. The ideal reactions involve
the magnesium endmember components of the olivine (reaction 1) or serpentine
(reaction 2), the latter derived from earlier olivine by hydration and
silicification (reaction 3). The presence of iron in the olivine or
serpentine reduces the efficiency of sequestration, since the iron
components of these minerals break down to iron oxide and silica
(reaction 4).
Serpentinite reactions
-
+ → + +(Reaction 1)
-
+ → + +(Reaction 2)
-
+ + →(Reaction 3)
-
+ → + +(Reaction 4)
Zeolitic imidazolate frameworks
Zeolitic imidazolate frameworks is a metal-organic framework carbon dioxide sink which could be used to keep industrial emissions of carbon dioxide out of the atmosphere.
Trends in sink performance
One
study in 2009 found that the fraction of fossil-fuel emissions absorbed
by the oceans may have declined by up to 10% since 2000, indicating
oceanic sequestration may be sublinear. Another 2009 study found that the fraction of CO2 absorbed by terrestrial ecosystems and the oceans has not changed since 1850, indicating undiminished capacity.