Estimated change in sea water pH caused by human created CO
2 between the 1700s and the 1990s, from the Global Ocean Data Analysis Project (GLODAP) and the World Ocean Atlas
2 between the 1700s and the 1990s, from the Global Ocean Data Analysis Project (GLODAP) and the World Ocean Atlas
NOAA
provides evidence for upwelling of "acidified" water onto the
Continental Shelf. In the figure above, note the vertical sections of
(A) temperature, (B) aragonite saturation, (C) pH, (D) DIC, and (E) pCO2
on transect line 5 off Pt. St. George, California. The potential
density surfaces are superimposed on the temperature section. The 26.2
potential density surface delineates the location of the first instance
in which the undersaturated water is upwelled from depths of 150 to
200 m onto the shelf and outcropping at the surface near the coast. The
red dots represent sample locations.
Ocean acidification is the ongoing decrease in the pH of the Earth's oceans, caused by the uptake of carbon dioxide (CO2) from the atmosphere. Seawater is slightly basic
(meaning the pH is greater than 7), and ocean acidification involves a shift towards
pH-neutral conditions rather than a transition to acidic conditions (pH less than 7).
An estimated 30–40% of the carbon dioxide from human activity released
into the atmosphere dissolves into oceans, rivers and lakes. To achieve chemical equilibrium, some of it reacts with the water to form carbonic acid. Some of the resulting carbonic acid molecules dissociate into a bicarbonate ion and a hydrogen ion, thus increasing ocean acidity (H+ ion concentration). Between 1751 and 1996, surface ocean pH is estimated to have decreased from approximately 8.25 to 8.14, representing an increase of almost 30% in H+ ion concentration in the world's oceans. Earth System Models project that, within the last decade, ocean acidity exceeded historical analogues and, in combination with other ocean biogeochemical
changes, could undermine the functioning of marine ecosystems and
disrupt the provision of many goods and services associated with the
ocean beginning as early as 2100.
Increasing acidity is thought to have a range of potentially
harmful consequences for marine organisms, such as depressing metabolic
rates and immune responses in some organisms, and causing coral bleaching.
By increasing the presence of free hydrogen ions, the additional
carbonic acid that forms in the oceans ultimately results in the
conversion of carbonate ions into bicarbonate ions. Ocean alkalinity (roughly equal to [HCO3−] + 2[CO32−]) is not changed by the process, or may increase over long time periods due to carbonate dissolution. This net decrease in the amount of carbonate ions available may make it more difficult for marine calcifying organisms, such as coral and some plankton, to form biogenic calcium carbonate, and such structures become vulnerable to dissolution. Ongoing acidification of the oceans may threaten future food chains linked with the oceans. As members of the InterAcademy Panel, 105 science academies have issued a statement on ocean acidification recommending that by 2050, global CO2 emissions be reduced by at least 50% compared to the 1990 level.
While ongoing ocean acidification is at least partially anthropogenic in origin, it has occurred previously in Earth's history. The most notable example is the Paleocene-Eocene Thermal Maximum (PETM),
which occurred approximately 56 million years ago when massive amounts
of carbon entered the ocean and atmosphere, and led to the dissolution
of carbonate sediments in all ocean basins.
Ocean acidification has been compared to anthropogenic climate change and called the "evil twin of global warming" and "the other CO2 problem". Freshwater bodies also appear to be acidifying, although this is a more complex and less obvious phenomenon.
Carbon cycle
The CO
2 cycle between the atmosphere and the ocean
2 cycle between the atmosphere and the ocean
The carbon cycle describes the fluxes of carbon dioxide (CO
2) between the oceans, terrestrial biosphere, lithosphere, and the atmosphere. Human activities such as the combustion of fossil fuels and land use changes have led to a new flux of CO
2 into the atmosphere. About 45% has remained in the atmosphere; most of the rest has been taken up by the oceans, with some taken up by terrestrial plants.
2) between the oceans, terrestrial biosphere, lithosphere, and the atmosphere. Human activities such as the combustion of fossil fuels and land use changes have led to a new flux of CO
2 into the atmosphere. About 45% has remained in the atmosphere; most of the rest has been taken up by the oceans, with some taken up by terrestrial plants.
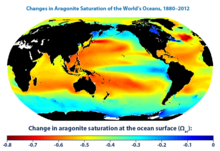
Distribution of (A) aragonite and (B) calcite saturation depth in the global oceans
The
map was created by the National Oceanic and Atmospheric Administration
and the Woods Hole Oceanographic Institution using Community Earth
System Model data. This map was created by comparing average conditions
during the 1880s with average conditions during the most recent 10 years
(2003–2012). Aragonite saturation has only been measured at selected
locations during the last few decades, but it can be calculated reliably
for different times and locations based on the relationships scientists
have observed among aragonite saturation, pH, dissolved carbon, water
temperature, concentrations of carbon dioxide in the atmosphere, and
other factors that can be measured. This map shows changes in the amount
of aragonite dissolved in ocean surface waters between the 1880s and
the most recent decade (2003–2012). Aragonite saturation is a ratio that
compares the amount of aragonite that is actually present with the
total amount of aragonite that the water could hold if it were
completely saturated. The more negative the change in aragonite
saturation, the larger the decrease in aragonite available in the water,
and the harder it is for marine creatures to produce their skeletons
and shells. The global map shows changes over time in the amount of
aragonite dissolved in ocean water, which is called aragonite
saturation.
The carbon cycle involves both organic compounds such as cellulose and inorganic carbon compounds such as carbon dioxide, carbonate ion, and bicarbonate ion.
The inorganic compounds are particularly relevant when discussing ocean
acidification for they include many forms of dissolved CO
2 present in the Earth's oceans.
2 present in the Earth's oceans.
When CO
2 dissolves, it reacts with water to form a balance of ionic and non-ionic chemical species: dissolved free carbon dioxide (CO
2(aq)), carbonic acid (H
2CO
3), bicarbonate (HCO−
3) and carbonate (CO2−
3). The ratio of these species depends on factors such as seawater temperature, pressure and salinity (as shown in a Bjerrum plot). These different forms of dissolved inorganic carbon are transferred from an ocean's surface to its interior by the ocean's solubility pump.
2 dissolves, it reacts with water to form a balance of ionic and non-ionic chemical species: dissolved free carbon dioxide (CO
2(aq)), carbonic acid (H
2CO
3), bicarbonate (HCO−
3) and carbonate (CO2−
3). The ratio of these species depends on factors such as seawater temperature, pressure and salinity (as shown in a Bjerrum plot). These different forms of dissolved inorganic carbon are transferred from an ocean's surface to its interior by the ocean's solubility pump.
The resistance of an area of ocean to absorbing atmospheric CO
2 is known as the Revelle factor.
2 is known as the Revelle factor.
Acidification
Dissolving CO
2 in seawater increases the hydrogen ion (H+) concentration in the ocean, and thus decreases ocean pH, as follows:
2 in seawater increases the hydrogen ion (H+) concentration in the ocean, and thus decreases ocean pH, as follows:
CO2 (aq) + H2O ⇌ H2CO3 ⇌ HCO3− + H+ ⇌ CO32− + 2 H+.
Caldeira and Wickett (2003) placed the rate and magnitude of modern ocean acidification changes in the context of probable historical changes during the last 300 million years.
Since the industrial revolution began, the ocean has absorbed about a third of the CO
2 we have produced since then and it is estimated that surface ocean pH has dropped by slightly more than 0.1 units on the logarithmic scale of pH, representing about a 29% increase in H+. It is expected to drop by a further 0.3 to 0.5 pH units (an additional doubling to tripling of today's post-industrial acid concentrations) by 2100 as the oceans absorb more anthropogenic CO
2, the impacts being most severe for coral reefs and the Southern Ocean. These changes are predicted to accelerate as more anthropogenic CO
2 is released to the atmosphere and taken up by the oceans. The degree of change to ocean chemistry, including ocean pH, will depend on the mitigation and emissions pathways taken by society.
2 we have produced since then and it is estimated that surface ocean pH has dropped by slightly more than 0.1 units on the logarithmic scale of pH, representing about a 29% increase in H+. It is expected to drop by a further 0.3 to 0.5 pH units (an additional doubling to tripling of today's post-industrial acid concentrations) by 2100 as the oceans absorb more anthropogenic CO
2, the impacts being most severe for coral reefs and the Southern Ocean. These changes are predicted to accelerate as more anthropogenic CO
2 is released to the atmosphere and taken up by the oceans. The degree of change to ocean chemistry, including ocean pH, will depend on the mitigation and emissions pathways taken by society.
Although the largest changes are expected in the future, a report from NOAA scientists found large quantities of water undersaturated in aragonite are already upwelling close to the Pacific continental shelf area of North America. Continental shelves play an important role in marine ecosystems since most marine organisms live or are spawned there, and though the study only dealt with the area from Vancouver to Northern California, the authors suggest that other shelf areas may be experiencing similar effects.
| Time | pH | pH change relative to pre-industrial |
Source | H+ concentration change relative to pre-industrial |
|---|---|---|---|---|
| Pre-industrial (18th century) | 8.179 | analyzed field | ||
| Recent past (1990s) | 8.104 | −0.075 | field | + 18.9% |
| Present levels | ~8.069 | −0.11 | field | + 28.8% |
| 2050 (2×CO 2 = 560 ppm) |
7.949 | −0.230 | model | + 69.8% |
| 2100 (IS92a) | 7.824 | −0.355 | model | + 126.5% |
Rate
One of the first detailed datasets to examine how pH varied over 8 years at a specific north temperate coastal location found that acidification had strong links to in situ
benthic species dynamics and that the variation in ocean pH may cause
calcareous species to perform more poorly than noncalcareous species in
years with low pH and predicts consequences for near-shore benthic ecosystems. Thomas Lovejoy,
former chief biodiversity advisor to the World Bank, has suggested that
"the acidity of the oceans will more than double in the next 40 years.
He says this rate is 100 times faster than any changes in ocean acidity
in the last 20 million years, making it unlikely that marine life can
somehow adapt to the changes."
It is predicted that, by the year 2100, If co-occurring biogeochemical
changes influence the delivery of ocean goods and services, then they
could also have a considerable effect on human welfare for those who
rely heavily on the ocean for food, jobs, and revenues.
Current rates of ocean acidification have been compared with the
greenhouse event at the Paleocene–Eocene boundary (about 55 million
years ago) when surface ocean temperatures rose by 5–6 degrees Celsius.
No catastrophe was seen in surface ecosystems, yet bottom-dwelling
organisms in the deep ocean experienced a major extinction. The current
acidification is on a path to reach levels higher than any seen in the
last 65 million years,
and the rate of increase is about ten times the rate that preceded the
Paleocene–Eocene mass extinction. The current and projected
acidification has been described as an almost unprecedented geological
event.
A National Research Council study released in April 2010 likewise
concluded that "the level of acid in the oceans is increasing at an
unprecedented rate". A 2012 paper in the journal Science
examined the geological record in an attempt to find a historical
analog for current global conditions as well as those of the future. The
researchers determined that the current rate of ocean acidification is
faster than at any time in the past 300 million years.
A review by climate scientists at the RealClimate blog, of a 2005 report by the Royal Society of the UK similarly highlighted the centrality of the rates of change in the present anthropogenic acidification process, writing:
The natural pH of the ocean is determined by a need to balance the deposition and burial of CaCO
3 on the sea floor against the influx of Ca2+ and CO2−
3 into the ocean from dissolving rocks on land, called weathering. These processes stabilize the pH of the ocean, by a mechanism called CaCO
3 compensation...The point of bringing it up again is to note that if the CO
2 concentration of the atmosphere changes more slowly than this, as it always has throughout the Vostok record, the pH of the ocean will be relatively unaffected because CaCO
3 compensation can keep up. The [present] fossil fuel acidification is much faster than natural changes, and so the acid spike will be more intense than the earth has seen in at least 800,000 years.
In the 15-year period 1995–2010 alone, acidity has increased 6
percent in the upper 100 meters of the Pacific Ocean from Hawaii to
Alaska. According to a statement in July 2012 by Jane Lubchenco, head of the U.S. National Oceanic and Atmospheric Administration
"surface waters are changing much more rapidly than initial
calculations have suggested. It's yet another reason to be very
seriously concerned about the amount of carbon dioxide that is in the
atmosphere now and the additional amount we continue to put out."
A 2013 study claimed acidity was increasing at a rate 10 times faster than in any of the evolutionary crises in Earth's history. In a synthesis report published in Science in 2015, 22 leading marine scientists stated that CO2 from burning fossil fuels is changing the oceans' chemistry more rapidly than at any time since the Great Dying,
Earth's most severe known extinction event, emphasizing that the 2 °C
maximum temperature increase agreed upon by governments reflects too
small a cut in emissions to prevent "dramatic impacts" on the world's
oceans, with lead author Jean-Pierre Gattuso remarking that "The ocean
has been minimally considered at previous climate negotiations. Our
study provides compelling arguments for a radical change at the UN
conference (in Paris) on climate change".
The rate at which ocean acidification will occur may be
influenced by the rate of surface ocean warming, because the chemical
equilibria that govern seawater pH are temperature-dependent. Greater seawater warming could lead to a smaller change in pH for a given increase in CO2.
Calcification
Overview
Changes
in ocean chemistry can have extensive direct and indirect effects on
organisms and their habitats. One of the most important repercussions of
increasing ocean acidity relates to the production of shells and plates
out of calcium carbonate (CaCO
3). This process is called calcification and is important to the biology and survival of a wide range of marine organisms. Calcification involves the precipitation of dissolved ions into solid CaCO
3 structures, such as coccoliths. After they are formed, such structures are vulnerable to dissolution unless the surrounding seawater contains saturating concentrations of carbonate ions (CO32−).
3). This process is called calcification and is important to the biology and survival of a wide range of marine organisms. Calcification involves the precipitation of dissolved ions into solid CaCO
3 structures, such as coccoliths. After they are formed, such structures are vulnerable to dissolution unless the surrounding seawater contains saturating concentrations of carbonate ions (CO32−).
Mechanism
Bjerrum plot: Change in carbonate system of seawater from ocean acidification.
Of the extra carbon dioxide added into the oceans, some remains as
dissolved carbon dioxide, while the rest contributes towards making
additional bicarbonate (and additional carbonic acid). This also
increases the concentration of hydrogen ions, and the percentage
increase in hydrogen is larger than the percentage increase in
bicarbonate, creating an imbalance in the reaction HCO3− ⇌ CO32− + H+.
To maintain chemical equilibrium, some of the carbonate ions already in
the ocean combine with some of the hydrogen ions to make further
bicarbonate. Thus the ocean's concentration of carbonate ions is
reduced, creating an imbalance in the reaction Ca2+ + CO32− ⇌ CaCO3, and making the dissolution of formed CaCO
3 structures more likely.
3 structures more likely.
The increase in concentrations of dissolved carbon dioxide and bicarbonate, and reduction in carbonate, are shown in a Bjerrum plot.
Saturation state
The saturation
state (known as Ω) of seawater for a mineral is a measure of the
thermodynamic potential for the mineral to form or to dissolve, and for
calcium carbonate is described by the following equation:
Here Ω is the product of the concentrations (or activities) of the reacting ions that form the mineral (Ca2+ and CO2−
3), divided by the product of the concentrations of those ions when the mineral is at equilibrium (K
sp), that is, when the mineral is neither forming nor dissolving. In seawater, a natural horizontal boundary is formed as a result of temperature, pressure, and depth, and is known as the saturation horizon. Above this saturation horizon, Ω has a value greater than 1, and CaCO
3 does not readily dissolve. Most calcifying organisms live in such waters. Below this depth, Ω has a value less than 1, and CaCO
3 will dissolve. However, if its production rate is high enough to offset dissolution, CaCO
3 can still occur where Ω is less than 1. The carbonate compensation depth occurs at the depth in the ocean where production is exceeded by dissolution.
3), divided by the product of the concentrations of those ions when the mineral is at equilibrium (K
sp), that is, when the mineral is neither forming nor dissolving. In seawater, a natural horizontal boundary is formed as a result of temperature, pressure, and depth, and is known as the saturation horizon. Above this saturation horizon, Ω has a value greater than 1, and CaCO
3 does not readily dissolve. Most calcifying organisms live in such waters. Below this depth, Ω has a value less than 1, and CaCO
3 will dissolve. However, if its production rate is high enough to offset dissolution, CaCO
3 can still occur where Ω is less than 1. The carbonate compensation depth occurs at the depth in the ocean where production is exceeded by dissolution.
The decrease in the concentration of CO32− decreases Ω, and hence makes CaCO
3 dissolution more likely.
3 dissolution more likely.
Calcium carbonate occurs in two common polymorphs (crystalline forms): aragonite and calcite.
Aragonite is much more soluble than calcite, so the aragonite
saturation horizon is always nearer to the surface than the calcite
saturation horizon.
This also means that those organisms that produce aragonite may be more
vulnerable to changes in ocean acidity than those that produce calcite. Increasing CO
2 levels and the resulting lower pH of seawater decreases the saturation state of CaCO
3 and raises the saturation horizons of both forms closer to the surface. This decrease in saturation state is believed to be one of the main factors leading to decreased calcification in marine organisms, as the inorganic precipitation of CaCO
3 is directly proportional to its saturation state.
2 levels and the resulting lower pH of seawater decreases the saturation state of CaCO
3 and raises the saturation horizons of both forms closer to the surface. This decrease in saturation state is believed to be one of the main factors leading to decreased calcification in marine organisms, as the inorganic precipitation of CaCO
3 is directly proportional to its saturation state.
Possible impacts
Increasing acidity has possibly harmful consequences, such as depressing metabolic rates in jumbo squid, depressing the immune responses of blue mussels, and coral bleaching. However it may benefit some species, for example increasing the growth rate of the sea star, Pisaster ochraceus, while shelled plankton species may flourish in altered oceans.
The report "Ocean Acidification Summary for Policymakers 2013" describes research findings and possible impacts.
Impacts on oceanic calcifying organisms
Although the natural absorption of CO
2 by the world's oceans helps mitigate the climatic effects of anthropogenic emissions of CO
2, it is believed that the resulting decrease in pH will have negative consequences, primarily for oceanic calcifying organisms. These span the food chain from autotrophs to heterotrophs and include organisms such as coccolithophores, corals, foraminifera, echinoderms, crustaceans and molluscs. As described above, under normal conditions, calcite and aragonite are stable in surface waters since the carbonate ion is at supersaturating concentrations. However, as ocean pH falls, the concentration of carbonate ions required for saturation to occur increases, and when carbonate becomes undersaturated, structures made of calcium carbonate are vulnerable to dissolution. Therefore, even if there is no change in the rate of calcification, the rate of dissolution of calcareous material increases.
2 by the world's oceans helps mitigate the climatic effects of anthropogenic emissions of CO
2, it is believed that the resulting decrease in pH will have negative consequences, primarily for oceanic calcifying organisms. These span the food chain from autotrophs to heterotrophs and include organisms such as coccolithophores, corals, foraminifera, echinoderms, crustaceans and molluscs. As described above, under normal conditions, calcite and aragonite are stable in surface waters since the carbonate ion is at supersaturating concentrations. However, as ocean pH falls, the concentration of carbonate ions required for saturation to occur increases, and when carbonate becomes undersaturated, structures made of calcium carbonate are vulnerable to dissolution. Therefore, even if there is no change in the rate of calcification, the rate of dissolution of calcareous material increases.
Corals, coccolithophore algae, coralline algae, foraminifera, shellfish, and pteropods experience reduced calcification or enhanced dissolution when exposed to elevated CO
2.
2.
The Royal Society published a comprehensive overview of ocean acidification, and its potential consequences, in June 2005.
However, some studies have found different response to ocean
acidification, with coccolithophore calcification and photosynthesis
both increasing under elevated atmospheric pCO2, an equal decline in primary production and calcification in response to elevated CO2 or the direction of the response varying between species. A study in 2008 examining a sediment core from the North Atlantic found that while the species composition of coccolithophorids has remained unchanged for the industrial period 1780 to 2004, the calcification of coccoliths has increased by up to 40% during the same time. A 2010 study from Stony Brook University
suggested that while some areas are overharvested and other fishing
grounds are being restored, because of ocean acidification it may be
impossible to bring back many previous shellfish populations.
While the full ecological consequences of these changes in
calcification are still uncertain, it appears likely that many
calcifying species will be adversely affected.
When exposed in experiments to pH reduced by 0.2 to 0.4, larvae of a temperate brittlestar, a relative of the common sea star, fewer than 0.1 percent survived more than eight days. There is also a suggestion that a decline in the coccolithophores may have secondary effects on climate, contributing to global warming by decreasing the Earth's albedo via their effects on oceanic cloud cover. All marine ecosystems on Earth will be exposed to changes in acidification and several other ocean biogeochemical changes.
The fluid in the internal compartments where corals grow their exoskeleton
is also extremely important for calcification growth. When the
saturation rate of aragonite in the external seawater is at ambient
levels, the corals will grow their aragonite crystals rapidly in their
internal compartments, hence their exoskeleton grows rapidly. If the
level of aragonite in the external seawater is lower than the ambient
level, the corals have to work harder to maintain the right balance in
the internal compartment. When that happens, the process of growing the
crystals slows down, and this slows down the rate of how much their
exoskeleton is growing. Depending on how much aragonite is in the
surrounding water, the corals may even stop growing because the levels
of aragonite are too low to pump into the internal compartment. They
could even dissolve faster than they can make the crystals to their
skeleton, depending on the aragonite levels in the surrounding water.
Under the current progression of carbon emissions, around 70% of North
Atlantic cold-water corals will be living in corrosive waters by
2050-60.
A study conducted by the Woods Hole Oceanographic Institution
in January 2018 showed that the skeletal growth of corals under
acidified conditions is primarily affected by a reduced capacity to
build dense exoskeletons, rather than affecting the linear extension of
the exoskeleton. Using Global Climate Models, they show that the
density of some species of corals could be reduced by over 20% by the
end of this century.
An in situ experiment on a 400 m2 patch of the Great Barrier Reef to decrease seawater CO2 level (raise pH) to close to the preindustrial value showed a 7% increase in net calcification.
A similar experiment to raise in situ seawater seawater CO2 level (lower pH) to a level expected soon after the middle of this century found that net calcification decreased 34%.
Ocean acidification may force some organisms to reallocate
resources away from productive endpoints such as growth in order to
maintain calcification.
In some places carbon dioxide bubbles out from the sea floor,
locally changing the pH and other aspects of the chemistry of the
seawater. Studies of these carbon dioxide seeps have documented a
variety of responses by different organisms.
Coral reef communities located near carbon dioxide seeps are of
particular interest because of the sensitivity of some corals species to
acidification. In Papua New Guinea, declining pH caused by carbon dioxide seeps is associated with declines in coral species diversity. However, in Palau
carbon dioxide seeps are not associated with reduced species diversity
of corals, although bioerosion of coral skeletons is much higher at low
pH sites.
Other biological impacts
Aside
from the slowing and/or reversing of calcification, organisms may
suffer other adverse effects, either indirectly through negative impacts
on food resources, or directly as reproductive or physiological effects. For example, the elevated oceanic levels of CO2 may produce CO
2-induced acidification of body fluids, known as hypercapnia. Also, increasing ocean acidity is believed to have a range of direct consequences. For example, increasing acidity has been observed to: reduce metabolic rates in jumbo squid; depress the immune responses of blue mussels; and make it harder for juvenile clownfish to tell apart the smells of non-predators and predators, or hear the sounds of their predators. This is possibly because ocean acidification may alter the acoustic properties of seawater, allowing sound to propagate further, and increasing ocean noise. This impacts all animals that use sound for echolocation or communication. Atlantic longfin squid eggs took longer to hatch in acidified water, and the squid's statolith was smaller and malformed in animals placed in sea water with a lower pH. The lower PH was simulated with 20-30 times the normal amount of CO2. However, as with calcification, as yet there is not a full understanding of these processes in marine organisms or ecosystems.
2-induced acidification of body fluids, known as hypercapnia. Also, increasing ocean acidity is believed to have a range of direct consequences. For example, increasing acidity has been observed to: reduce metabolic rates in jumbo squid; depress the immune responses of blue mussels; and make it harder for juvenile clownfish to tell apart the smells of non-predators and predators, or hear the sounds of their predators. This is possibly because ocean acidification may alter the acoustic properties of seawater, allowing sound to propagate further, and increasing ocean noise. This impacts all animals that use sound for echolocation or communication. Atlantic longfin squid eggs took longer to hatch in acidified water, and the squid's statolith was smaller and malformed in animals placed in sea water with a lower pH. The lower PH was simulated with 20-30 times the normal amount of CO2. However, as with calcification, as yet there is not a full understanding of these processes in marine organisms or ecosystems.
Another possible effect would be an increase in red tide events, which could contribute to the accumulation of toxins (domoic acid, brevetoxin, saxitoxin) in small organisms such as anchovies and shellfish, in turn increasing occurrences of amnesic shellfish poisoning, neurotoxic shellfish poisoning and paralytic shellfish poisoning.
Ecosystem impacts amplified by ocean warming and deoxygenation
While the full implications of elevated CO2
on marine ecosystems are still being documented, there is a substantial
body of research showing that a combination of ocean acidification and
elevated ocean temperature, driven mainly by CO2 and other
greenhouse gas emissions, have a compounded effect on marine life and
the ocean environment. This effect far exceeds the individual harmful
impact of either. In addition, ocean warming exacerbates ocean deoxygenation,
which is an additional stressor on marine organisms, by increasing
ocean stratification, through density and solubility effects, thus
limiting nutrients, while at the same time increasing metabolic demand.
Meta analyses have quantified the direction and magnitude of the
harmful effects of ocean acidification, warming and deoxygenation on the
ocean. These meta-analyses have been further tested by mesocosm studies
that simulated the interaction of these stressors and found a
catastrophic effect on the marine food web, i.e. that the increases in
consumption from thermal stress more than negates any primary producer
to herbivore increase from elevated CO2.
Nonbiological impacts
Leaving
aside direct biological effects, it is expected that ocean
acidification in the future will lead to a significant decrease in the
burial of carbonate sediments for several centuries, and even the
dissolution of existing carbonate sediments. This will cause an elevation of ocean alkalinity, leading to the enhancement of the ocean as a reservoir for CO2 with implications for climate change as more CO2 leaves the atmosphere for the ocean.
Impact on human industry
The threat of acidification includes a decline in commercial fisheries
and in the Arctic tourism industry and economy. Commercial fisheries
are threatened because acidification harms calcifying organisms which
form the base of the Arctic food webs.
Pteropods and brittle stars both form the base of the Arctic food webs
and are both seriously damaged from acidification. Pteropods shells
dissolve with increasing acidification and the brittle stars lose muscle
mass when re-growing appendages.
For pteropods to create shells they require aragonite which is produced
through carbonate ions and dissolved calcium. Pteropods are severely
affected because increasing acidification levels have steadily decreased
the amount of water supersaturated with carbonate which is needed for
aragonite creation. Arctic waters are changing so rapidly that they will become undersaturated with aragonite as early as 2016.
Additionally the brittle star's eggs die within a few days when exposed
to expected conditions resulting from Arctic acidification.
Acidification threatens to destroy Arctic food webs from the base up.
Arctic food webs are considered simple, meaning there are few steps in
the food chain from small organisms to larger predators. For example,
pteropods are "a key prey item of a number of higher predators – larger
plankton, fish, seabirds, whales".
Both pteropods and sea stars serve as a substantial food source and
their removal from the simple food web would pose a serious threat to
the whole ecosystem. The effects on the calcifying organisms at the base
of the food webs could potentially destroy fisheries. The value of fish
caught from US commercial fisheries in 2007 was valued at $3.8 billion
and of that 73% was derived from calcifiers and their direct predators.
Other organisms are directly harmed as a result of acidification. For
example, decrease in the growth of marine calcifiers such as the American lobster, ocean quahog, and scallops means there is less shellfish meat available for sale and consumption.
Red king crab fisheries are also at a serious threat because crabs are
calcifiers and rely on carbonate ions for shell development. Baby red
king crab when exposed to increased acidification levels experienced
100% mortality after 95 days.
In 2006, red king crab accounted for 23% of the total guideline harvest
levels and a serious decline in red crab population would threaten the
crab harvesting industry.
Several ocean goods and services are likely to be undermined by future
ocean acidification potentially affecting the livelihoods of some 400
to 800 million people depending upon the emission scenario.
Impact on indigenous peoples
Acidification
could damage the Arctic tourism economy and affect the way of life of
indigenous peoples. A major pillar of Arctic tourism is the sport
fishing and hunting industry. The sport fishing industry is threatened
by collapsing food webs which provide food for the prized fish. A
decline in tourism lowers revenue input in the area, and threatens the
economies that are increasingly dependent on tourism. The rapid decrease or disappearance of marine life could also affect the diet of Indigenous peoples.
Ocean Acidification in the Arctic Ocean
Annual Arctic Sea Ice Minimum
The Arctic Ocean has experienced drastic change over the years due to global warming.
It has been known that the Arctic Ocean acidity levels have been
increasing and at twice the rate compared to the Pacific and Atlantic
oceans. The loss of sea ice
has been connected to a decrease in pH levels in the ocean water. Sea
ice has experienced an extreme reduction over the past 30 years, forming
a minimum area of 2.9×106 km2 at the end of the boreal summer of 2007, 47%, less than in 1980. Sea ice limits the air-sea gas exchange
with carbon dioxide. With less water completely exposed to the
atmosphere, the levels of carbon dioxide gas in the water remain low.
The Arctic Ocean should have low carbon dioxide levels due to intense
cooling, run off of fresh water and photosynthesis from marine
organisms.
However, the decrease of sea ice over the years due to global warming
has limited freshwater runoff and has exposed a higher percentage of the
ocean surface to the atmosphere. The increase of carbon dioxide in the
water decreases the pH of the ocean causing ocean acidification.
The decrease in sea ice has also allowed more Pacific water to flow
into in the Arctic Ocean during the winter, this is called Pacific
winter water.
The Pacific water flows into the Arctic Ocean carrying additional
amounts of carbon dioxide by being exposed to the atmosphere and
absorbing carbon dioxide from decaying organic matter and from
sediments.
The Arctic Ocean pH levels are rapidly decreasing because not
only is the ocean water absorbing more carbon dioxide due to increased
surface area exposure as a result of a decrease in sea ice. It also has
large amounts of carbon dioxide being transferred to the Arctic from the
Pacific ocean.
Cold water is able to absorb higher amounts of carbon dioxide
compared to warm water. The solubility of gases decreases in relation to
increasing temperature. Cold water bodies are absorbing the increasing
amount of carbon dioxide in the atmosphere and becoming known as carbon
sinks.
The increasing amount of carbon dioxide in the water is putting many
organisms at risk as they are affected by the increase of acidity in the
ocean water.
Effects of Ocean Acidification on Arctic Organisms
Organisms
in Arctic waters are already challenged with stressors of living in the
Arctic Ocean, such as dealing with cold temperatures, and it is thought
that because of this, additional stressors such as ocean acidification,
will cause ocean acidification effects on marine organisms to appear
first in the Arctic. There exists a significant variation in the
sensitivity of marine organisms to increased ocean acidification.
Calcifying organisms generally exhibit larger negative responses from
ocean acidification than non‐calcifying organisms across numerous
response variables, with the exception of crustaceans, which calcify but
were not negatively affected. The acidification of the Arctic Ocean will impact these marine calcifiers in several different ways.
The uptake of CO₂ by seawater increases the concentration of hydrogen
ions, which lowers pH and, in changing the chemical equilibrium of the
inorganic carbon system, reduces the concentration of carbonate ions
(CO₃²⁻).
Carbonate ions are required by marine calcifying organisms such as
plankton, shellfish, and fish to produce calcium carbonate (CaCO₃)
shells and skeletons.
Arctic Council map
For either aragonite or calcite, the two polymorphs of CaCO₃ produced by marine organisms, the saturation state of CaCO₃ in ocean water is expressed by the product of the concentrations of CO₂²⁻ and Ca²⁺ in seawater relative to the stoichiometric solubility product at a given temperature, salinity, and pressure. Waters which are saturated in CaCO₃ are favorable to precipitation and formation of CaCO₃ shells and skeletons, but waters which are undersaturated are corrosive to CaCO₃ shells, and in the absence of protective mechanisms, dissolution of calcium carbonate will occur. Because colder arctic water absorbs more CO₂, the concentration of CO₃²⁻ is reduced, therefore the saturation of calcium carbonate is lower in high-latitude oceans than it is in tropical or temperate oceans. In model simulations of the Arctic Ocean, it is predicted that aragonite saturation will decrease, because of an increased amount of freshwater input from melting sea ice and increased carbon uptake as a result of sea ice retreat. This simulation predicts that Arctic surface waters will become undersaturated with aragonite within a decade. The undersaturation of aragonite will cause the shells of organisms which are constructed from aragonite to dissolve. This would have a profound effect on a large variety of marine organisms and has the potential to do devastating damage to keystone species and to the marine food web in the Arctic Ocean. Laboratory experiments on various marine biota in an elevated CO₂ environment show that changes in aragonite saturation cause substantial changes in overall calcification rates for many species of marine organisms, including coccolithophore, foraminifera, pteropods, mussels, and clams.
Although the undersaturation of arctic water has been proven to
have an effect on the ability of organisms to precipitate their shells,
recent studies have shown that the calcification rate of calcifiers,
such as corals,
coccolithophores, foraminiferans and bivalves, decrease with increasing
pCO₂, even in seawater supersaturated with respect to CaCO₃.
Additionally, increased pCO₂ has been found to have complex effects on
the physiology, growth and reproductive success of various marine
calcifiers.
CO₂ tolerance seems to differ between various marine organisms, as well
as differences in CO₂ tolerance at different life cycle stages (e.g.
larva and adult). The first stage in the life cycle of marine calcifiers
which are at a serious risk by high CO2 content is the planktonic
larval stage. The larval development of several marine species,
primarily sea urchins and bivalves, are highly affected by elevations of
seawater pCO₂.
In laboratory tests, numerous sea urchin embryos were reared under
different CO₂ concentrations until they developed to the larval stage.
It was found that once reaching this stage, larval and arm sizes were
significantly smaller, as well as abnormal skeleton morphology was noted
with increasing pCO₂.
Pterapod shell dissolved in seawater adjusted to an ocean chemistry projected for the year 2100
Similar findings have been found in CO₂ treated-mussel larvae, which showed a larval size decrease of about 20% and showed morphological abnormalities such as convex hinges, weaker and thinner shells and protrusion of mantle. The larval body size also impacts the encounter and clearance rates of food particles, and if larval shells are smaller or deformed, these larvae are more prone to starvation. In addition, CaCO₃ structures also serve vital functions for calcified larvae, such as defence against predation, as well as roles in feeding, buoyancy control and pH regulation. Another example of a species which may be seriously impacted by ocean acidification is Pteropods, which are shelled pelagic molluscs which play an important role in the food-web of various ecosystems. Since they harbor an aragonitic shell, they could be very sensitive to ocean acidification driven by the increase of anthropogenic CO₂ emissions.Laboratory tests showed that calcification exhibits a 28% decrease at the pH value of the Arctic Ocean expected for the year 2100, compared to the present pH value. This 28% decline of calcification in the lower pH condition is within the range reported also for other calcifying organisms such as corals. In contrast with sea urchin and bivalve larvae, corals and marine shrimps are more severely impacted by ocean acidification after settlement, while they developed into the polyp stage. From laboratory tests, the morphology of the CO₂-treated polyp endoskeleton of corals was disturbed and malformed compared to the radial pattern of control polyps.
This variability in the impact of ocean acidification on
different life cycle stages of different organisms can be partially
explained by the fact that most echinoderms and mollusks start shell and
skeleton synthesis at their larval stage, whereas corals start at the
settlement stage. Hence, these stages are highly susceptible to the
potential effects of ocean acidification.
Most calcifiers, such as corals, echinoderms, bivalves and crustaceans,
play important roles in coastal ecosystems as keystone species,
bioturbators and ecosystem engineers. The food web
in the Arctic Ocean is somewhat truncated, meaning it is short and
simple. Any impacts to key species in the food web can cause
exponentially devastating effects on the rest on the food chain as a
whole, as they will no longer have a reliable food source. If these
larger organisms no longer have any source of nutrients, they too will
eventually die off, and the entire Arctic Ocean ecosystem will be
affected. This would have a huge impact on the Arctic people who catch
Arctic fish for a living, as well as the economic repercussions which
would follow such a major shortage of food and living income for these
families.
Possible responses
Demonstrator calling for action against ocean acidification at the People's Climate March (2017).
Reducing CO2 emissions
Members of the InterAcademy Panel recommended that by 2050, global anthropogenic CO2 emissions be reduced less than 50% of the 1990 level. The 2009 statement also called on world leaders to:
- Acknowledge that ocean acidification is a direct and real consequence of increasing atmospheric CO2 concentrations, is already having an effect at current concentrations, and is likely to cause grave harm to important marine ecosystems as CO2 concentrations reach 450 [parts-per-million (ppm)] and above;
- ... Recognise that reducing the build up of CO2 in the atmosphere is the only practicable solution to mitigating ocean acidification;
- ... Reinvigorate action to reduce stressors, such as overfishing and pollution, on marine ecosystems to increase resilience to ocean acidification.
Stabilizing atmospheric CO2 concentrations at 450 ppm would require near-term emissions reductions, with steeper reductions over time.
The German Advisory Council on Global Change stated:
In order to prevent disruption of the calcification of marine organisms and the resultant risk of fundamentally altering marine food webs, the following guard rail should be obeyed: the pH of near surface waters should not drop more than 0.2 units below the pre-industrial average value in any larger ocean region (nor in the global mean).
One policy target related to ocean acidity is the magnitude of future global warming. Parties to the United Nations Framework Convention on Climate Change (UNFCCC) adopted a target of limiting warming to below 2 °C, relative to the pre-industrial level. Meeting this target would require substantial reductions in anthropogenic CO2 emissions.
Limiting global warming to below 2 °C would imply a reduction in
surface ocean pH of 0.16 from pre-industrial levels. This would
represent a substantial decline in surface ocean pH.
On September 25, 2015, USEPA denied a June 30, 2015, citizens petition that asked EPA to regulate CO2 under TSCA
in order to mitigate ocean acidification. In the denial, EPA said that
risks from ocean acidification were being "more efficiently and
effectively addressed" under domestic actions, e.g., under the Presidential Climate Action Plan,
and that multiple avenues are being pursued to work with and in other
nations to reduce emissions and deforestation and promote clean energy
and energy efficiency.
On March 28, 2017 the US by executive order rescinded the Climate Action Plan. On June 1, 2017 it was announced the US would withdraw from the Paris accords, and on June 12, 2017 that the US would abstain from the G7 Climate Change Pledge, two major international efforts to reduce CO2 emissions.
Climate engineering
Climate engineering
(mitigating temperature or pH effects of emissions) has been proposed
as a possible response to ocean acidification. The IAP (2009) statement cautioned against climate engineering as a policy response:
Mitigation approaches such as adding chemicals to counter the effects of acidification are likely to be expensive, only partly effective and only at a very local scale, and may pose additional unanticipated risks to the marine environment. There has been very little research on the feasibility and impacts of these approaches. Substantial research is needed before these techniques could be applied.
Reports by the WGBU (2006), the UK's Royal Society (2009), and the US National Research Council (2011) warned of the potential risks and difficulties associated with climate engineering.
Iron fertilization
Iron fertilization of the ocean could stimulate photosynthesis in phytoplankton. The phytoplankton would convert the ocean's dissolved carbon dioxide into carbohydrate
and oxygen gas, some of which would sink into the deeper ocean before
oxidizing. More than a dozen open-sea experiments confirmed that adding
iron to the ocean increases photosynthesis in phytoplankton by up to 30 times.
While this approach has been proposed as a potential solution to the
ocean acidification problem, mitigation of surface ocean acidification
might increase acidification in the less-inhabited deep ocean.
A report by the UK's Royal Society (2009)
reviewed the approach for effectiveness, affordability, timeliness and
safety. The rating for affordability was "medium", or "not expected to
be very cost-effective". For the other three criteria, the ratings
ranged from "low" to "very low" (i.e., not good). For example, in
regards to safety, the report found a "[high] potential for undesirable
ecological side effects", and that ocean fertilization "may increase
anoxic regions of ocean ('dead zones')".





![{\displaystyle {\Omega }={\frac {\left[{\ce {Ca^2+}}\right]\left[{\ce {CO3^2-}}\right]}{K_{sp}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0422ec8bbb280ae8547a20d486b928b0c21846f4)