The abundance of the chemical elements is a measure of the occurrence of the chemical elements relative to all other elements in a given environment. Abundance is measured in one of three ways: by the mass-fraction (the same as weight fraction); by the mole-fraction (fraction of atoms by numerical count, or sometimes fraction of molecules in gases); or by the volume-fraction.
Volume-fraction is a common abundance measure in mixed gases such as
planetary atmospheres, and is similar in value to molecular
mole-fraction for gas mixtures at relatively low densities and
pressures, and ideal gas mixtures. Most abundance values in this article are given as mass-fractions.
For example, the abundance of oxygen in pure water can be measured in two ways: the mass fraction is about 89%, because that is the fraction of water's mass which is oxygen. However, the mole-fraction is 33.3333...% because only 1 atom of 3 in water, H2O, is oxygen. As another example, looking at the mass-fraction abundance of hydrogen and helium in both the Universe as a whole and in the atmospheres of gas-giant planets such as Jupiter, it is 74% for hydrogen and 23–25% for helium; while the (atomic) mole-fraction for hydrogen is 92%, and for helium is 8%, in these environments. Changing the given environment to Jupiter's outer atmosphere, where hydrogen is diatomic while helium is not, changes the molecular
mole-fraction (fraction of total gas molecules), as well as the
fraction of atmosphere by volume, of hydrogen to about 86%, and of
helium to 13%.
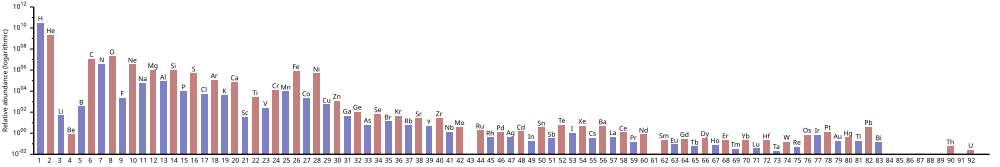
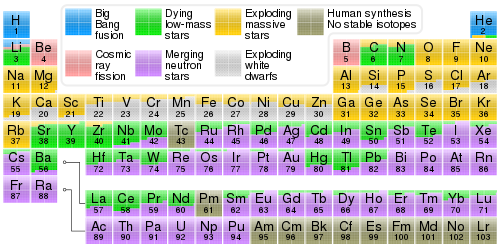
The abundance of chemical elements in the universe is dominated by the large amounts of hydrogen and helium which were produced in the Big Bang. Remaining elements, making up only about 2% of the universe, were largely produced by supernovae and certain red giant stars. Lithium, beryllium and boron are rare because although they are produced by nuclear fusion, they are then destroyed by other reactions in the stars. The elements from carbon to iron are relatively more common in the universe because of the ease of making them in supernova nucleosynthesis. Elements of higher atomic number than iron (element 26) become progressively more rare in the universe, because they increasingly absorb stellar energy in being produced. Elements with even atomic numbers are generally more common than their neighbors in the periodic table, also due to favorable energetics of formation.
The abundance of elements in the Sun and outer planets is similar to that in the universe. Due to solar heating, the elements of Earth and the inner rocky planets of the Solar System have undergone an additional depletion of volatile hydrogen, helium, neon, nitrogen, and carbon (which volatilizes as methane). The crust, mantle, and core of the Earth show evidence of chemical segregation plus some sequestration by density. Lighter silicates of aluminum are found in the crust, with more magnesium silicate in the mantle, while metallic iron and nickel compose the core. The abundance of elements in specialized environments, such as atmospheres, or oceans, or the human body, are primarily a product of chemical interactions with the medium in which they reside.
The abundance of chemical elements in the universe is dominated by the large amounts of hydrogen and helium which were produced in the Big Bang. Remaining elements, making up only about 2% of the universe, were largely produced by supernovae and certain red giant stars. Lithium, beryllium and boron are rare because although they are produced by nuclear fusion, they are then destroyed by other reactions in the stars. The elements from carbon to iron are relatively more common in the universe because of the ease of making them in supernova nucleosynthesis. Elements of higher atomic number than iron (element 26) become progressively more rare in the universe, because they increasingly absorb stellar energy in being produced. Elements with even atomic numbers are generally more common than their neighbors in the periodic table, also due to favorable energetics of formation.
The abundance of elements in the Sun and outer planets is similar to that in the universe. Due to solar heating, the elements of Earth and the inner rocky planets of the Solar System have undergone an additional depletion of volatile hydrogen, helium, neon, nitrogen, and carbon (which volatilizes as methane). The crust, mantle, and core of the Earth show evidence of chemical segregation plus some sequestration by density. Lighter silicates of aluminum are found in the crust, with more magnesium silicate in the mantle, while metallic iron and nickel compose the core. The abundance of elements in specialized environments, such as atmospheres, or oceans, or the human body, are primarily a product of chemical interactions with the medium in which they reside.
Universe
| Z | Element | Mass fraction (ppm) |
|---|---|---|
| 1 | Hydrogen | 739,000 |
| 2 | Helium | 240,000 |
| 8 | Oxygen | 10,400 |
| 6 | Carbon | 4,600 |
| 10 | Neon | 1,340 |
| 26 | Iron | 1,090 |
| 7 | Nitrogen | 960 |
| 14 | Silicon | 650 |
| 12 | Magnesium | 580 |
| 16 | Sulfur | 440 |
The elements – that is, ordinary (baryonic) matter made of protons, neutrons, and electrons, are only a small part of the content of the Universe. Cosmological observations
suggest that only 4.6% of the universe's energy (including the mass
contributed by energy, E = mc² ↔ m = E / c²) comprises the visible baryonic matter that constitutes stars, planets, and living beings. The rest is thought to be made up of dark energy (68%) and dark matter (27%). These are forms of matter and energy believed to exist on the basis of scientific theory and observational deductions, but they have not been directly observed and their nature is not well understood.
Most standard (baryonic) matter is found in intergalactic gas, stars, and interstellar clouds, in the form of atoms or ions (plasma), although it can be found in degenerate forms in extreme astrophysical settings, such as the high densities inside white dwarfs and neutron stars.
Hydrogen is the most abundant element in the Universe; helium is second. However, after this, the rank of abundance does not continue to correspond to the atomic number; oxygen has abundance rank 3, but atomic number 8. All others are substantially less common.
The abundance of the lightest elements is well predicted by the standard cosmological model, since they were mostly produced shortly (i.e., within a few hundred seconds) after the Big Bang, in a process known as Big Bang nucleosynthesis. Heavier elements were mostly produced much later, inside of stars.
Hydrogen and helium are estimated to make up roughly 74% and 24%
of all baryonic matter in the universe respectively. Despite comprising
only a very small fraction of the universe, the remaining "heavy
elements" can greatly influence astronomical phenomena. Only about 2%
(by mass) of the Milky Way galaxy's disk is composed of heavy elements.
These other elements are generated by stellar processes. In astronomy,
a "metal" is any element other than hydrogen or helium. This
distinction is significant because hydrogen and helium are the only
elements that were produced in significant quantities in the Big Bang.
Thus, the metallicity of a galaxy or other object is an indication of stellar activity, after the Big Bang.
In general, elements up to iron are made in large stars in the process of becoming supernovae. Iron-56
is particularly common, since it is the most stable element that can
easily be made from alpha particles (being a product of decay of
radioactive nickel-56,
ultimately made from 14 helium nuclei). Elements heavier than iron are
made in energy-absorbing processes in large stars, and their abundance
in the universe (and on Earth) generally decreases with increasing
atomic number.
Periodic table showing the cosmogenic origin of each element.
Solar system
| Nuclide | A | Mass fraction in parts per million | Atom fraction in parts per million |
|---|---|---|---|
| Hydrogen-1 | 1 | 705,700 | 909,964 |
| Helium-4 | 4 | 275,200 | 88,714 |
| Oxygen-16 | 16 | 9,592 | 477 |
| Carbon-12 | 12 | 3,032 | 326 |
| Nitrogen-14 | 14 | 1,105 | 102 |
| Neon-20 | 20 | 1,548 | 100 |
| Other nuclides: | 3,879 | 149 | |
| Silicon-28 | 28 | 653 | 30 |
| Magnesium-24 | 24 | 513 | 28 |
| Iron-56 | 56 | 1,169 | 27 |
| Sulfur-32 | 32 | 396 | 16 |
| Helium-3 | 3 | 35 | 15 |
| Hydrogen-2 | 2 | 23 | 15 |
| Neon-22 | 22 | 208 | 12 |
| Magnesium-26 | 26 | 79 | 4 |
| Carbon-13 | 13 | 37 | 4 |
| Magnesium-25 | 25 | 69 | 4 |
| Aluminium-27 | 27 | 58 | 3 |
| Argon-36 | 36 | 77 | 3 |
| Calcium-40 | 40 | 60 | 2 |
| Sodium-23 | 23 | 33 | 2 |
| Iron-54 | 54 | 72 | 2 |
| Silicon-29 | 29 | 34 | 2 |
| Nickel-58 | 58 | 49 | 1 |
| Silicon-30 | 30 | 23 | 1 |
| Iron-57 | 57 | 28 | 1 |
The above graph (note log scale) shows abundance of elements in the Solar System.
The table shows the twelve most common elements in our galaxy
(estimated spectroscopically), as measured in parts per million, by
mass.
Nearby galaxies that have evolved along similar lines have a
corresponding enrichment of elements heavier than hydrogen and helium.
The more distant galaxies are being viewed as they appeared in the past,
so their abundances of elements appear closer to the primordial
mixture. Since physical laws and processes are uniform throughout the
universe, however, it is expected that these galaxies will likewise have
evolved similar abundances of elements.
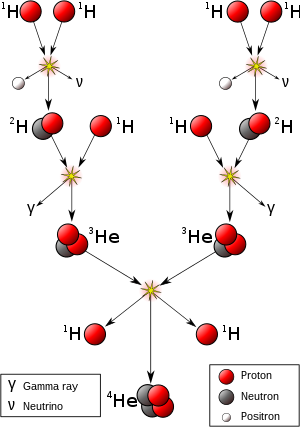
The abundance of elements is in keeping with their origin from the Big Bang and nucleosynthesis in a number of progenitor supernova
stars. Very abundant hydrogen and helium are products of the Big Bang,
while the next three elements are rare since they had little time to
form in the Big Bang and are not made in stars (they are, however,
produced in small quantities by breakup of heavier elements in
interstellar dust, as a result of impact by cosmic rays).
Beginning with carbon, elements have been produced in stars by buildup from alpha particles
(helium nuclei), resulting in an alternatingly larger abundance of
elements with even atomic numbers (these are also more stable). The
effect of odd-numbered chemical elements generally being more rare in
the universe was empirically noticed in 1914, and is known as the Oddo-Harkins rule.
Estimated abundances of the chemical elements in the Solar System (logarithmic scale).
Relation to nuclear binding energy
Loose correlations have been observed between estimated elemental abundances in the universe and the nuclear binding energy curve.
Roughly speaking, the relative stability of various atomic nuclides
has exerted a strong influence on the relative abundance of elements
formed in the Big Bang, and during the development of the universe thereafter.
See the article about nucleosynthesis for the explanation on how certain nuclear fusion processes in stars (such as carbon burning, etc.) create the elements heavier than hydrogen and helium.
A further observed peculiarity is the jagged alternation between
relative abundance and scarcity of adjacent atomic numbers in the
elemental abundance curve, and a similar pattern of energy levels in the
nuclear binding energy curve. This alternation is caused by the higher
relative binding energy (corresponding to relative stability) of even atomic numbers compared with odd atomic numbers and is explained by the Pauli Exclusion Principle.
The semi-empirical mass formula (SEMF), also called Weizsäcker's formula or the Bethe-Weizsäcker mass formula, gives a theoretical explanation of the overall shape of the curve of nuclear binding energy.
Earth
The Earth formed from the same cloud of matter that formed the Sun, but the planets acquired different compositions during the formation and evolution of the solar system. In turn, the natural history of the Earth caused parts of this planet to have differing concentrations of the elements.
The mass of the Earth is approximately 5.98×1024 kg. In bulk, by mass, it is composed mostly of iron (32.1%), oxygen (30.1%), silicon (15.1%), magnesium (13.9%), sulfur (2.9%), nickel (1.8%), calcium (1.5%), and aluminium (1.4%); with the remaining 1.2% consisting of trace amounts of other elements.
The bulk composition of the Earth by elemental-mass is roughly
similar to the gross composition of the solar system, with the major
differences being that Earth is missing a great deal of the volatile
elements hydrogen, helium, neon, and nitrogen, as well as carbon which
has been lost as volatile hydrocarbons. The remaining elemental
composition is roughly typical of the "rocky" inner planets, which
formed in the thermal zone where solar heat drove volatile compounds
into space. The Earth retains oxygen as the second-largest component of
its mass (and largest atomic-fraction), mainly from this element being
retained in silicate minerals which have a very high melting point and low vapor pressure.
Crust
Abundance
(atom fraction) of the chemical elements in Earth's upper continental
crust as a function of atomic number. The rarest elements in the crust
(shown in yellow) are rare due to a combination of factors: all but 1 of
them are the densest siderophiles (iron-loving) elements in the Goldschmidt classification
of elements, meaning they have a tendency to mix well with iron,
depleting them by being relocated deeper into the Earth's core. Their
abundance in meteoroids is higher. Additionally, tellurium has been depleted from the crust due to formation of volatile hydrides.
The mass-abundance of the nine most abundant elements in the Earth's
crust is approximately: oxygen 46%, silicon 28%, aluminum 8.2%, iron
5.6%, calcium 4.2%, sodium 2.5%, magnesium 2.4%, potassium 2.0%, and
titanium 0.61%. Other elements occur at less than 0.15%. For a complete
list, see abundance of elements in Earth's crust.
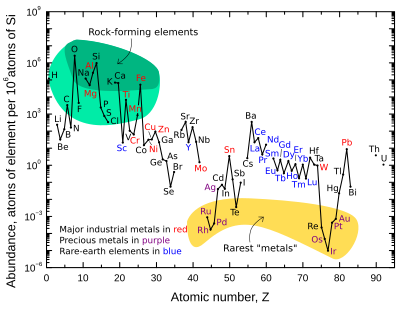
The graph above illustrates the relative atomic-abundance of
the chemical elements in Earth's upper continental crust— the part that
is relatively accessible for measurements and estimation.
Many of the elements shown in the graph are classified into (partially overlapping) categories:
- rock-forming elements (major elements in green field, and minor elements in light green field);
- rare earth elements (lanthanides, La-Lu, and Y; labeled in blue);
- major industrial metals (global production >~3×107 kg/year; labeled in red);
- precious metals (labeled in purple);
- the nine rarest "metals" — the six platinum group elements plus Au, Re, and Te (a metalloid) — in the yellow field. These are rare in the crust from being soluble in iron and thus concentrated in the Earth's core.
Note that there are two breaks where the unstable (radioactive) elements technetium (atomic number 43) and promethium
(atomic number 61) would be. These elements are surrounded by stable
elements, yet both have relatively short half lives (~ 4 million years
and ~ 18 years respectively). These are thus extremely rare, since any
primordial initial fractions of these in pre-Solar System materials have
long since decayed. These two elements are now only produced naturally
through the spontaneous fission of very heavy radioactive elements (for example, uranium, thorium, or the trace amounts of plutonium that exist in uranium ores), or by the interaction of certain other elements with cosmic rays.
Both technetium and promethium have been identified spectroscopically
in the atmospheres of stars, where they are produced by ongoing
nucleosynthetic processes.
There are also breaks in the abundance graph where the six noble gases
would be, since they are not chemically bound in the Earth's crust, and
they are only generated by decay chains from radioactive elements in
the crust, and are therefore extremely rare there.
The eight naturally occurring very rare, highly radioactive elements (polonium, astatine, francium, radium, actinium, protactinium, neptunium, and plutonium)
are not included, since any of these elements that were present at the
formation of the Earth have decayed away eons ago, and their quantity
today is negligible and is only produced from the radioactive decay of uranium and thorium.
Oxygen and silicon
are notably the most common elements in the crust. On Earth and in
rocky planets in general, silicon and oxygen are far more common than
their cosmic abundance. The reason is that they combine with each other
to form silicate minerals.
In this way, they are the lightest of all of the two-percent
"astronomical metals" (i.e., non-hydrogen and helium elements) to form a
solid that is refractory to the Sun's heat, and thus cannot boil away
into space. All elements lighter than oxygen have been removed from the
crust in this way.
Rare-earth elements
"Rare"
earth elements is a historical misnomer. The persistence of the term
reflects unfamiliarity rather than true rarity. The more abundant rare earth elements
are similarly concentrated in the crust compared to commonplace
industrial metals such as chromium, nickel, copper, zinc, molybdenum,
tin, tungsten, or lead. The two least abundant rare earth elements (thulium and lutetium)
are nearly 200 times more common than gold. However, in contrast to the
ordinary base and precious metals, rare earth elements have very little
tendency to become concentrated in exploitable ore deposits.
Consequently, most of the world's supply of rare earth elements comes
from only a handful of sources. Furthermore, the rare earth metals are
all quite chemically similar to each other, and they are thus quite
difficult to separate into quantities of the pure elements.
Differences in abundances of individual rare earth elements in
the upper continental crust of the Earth represent the superposition of
two effects, one nuclear and one geochemical. First, the rare earth
elements with even atomic numbers (58Ce, 60Nd, ...) have greater cosmic and terrestrial abundances than the adjacent rare earth elements with odd atomic numbers (57La, 59Pr,
...). Second, the lighter rare earth elements are more incompatible
(because they have larger ionic radii) and therefore more strongly
concentrated in the continental crust than the heavier rare earth
elements. In most rare earth ore deposits, the first four rare earth
elements – lanthanum, cerium, praseodymium, and neodymium – constitute 80% to 99% of the total amount of rare earth metal that can be found in the ore.
Mantle
The mass-abundance of the eight most abundant elements in the Earth's
mantle is approximately: oxygen 45%, magnesium
23%, silicon 22%, iron 5.8%, calcium 2.3%, aluminum 2.2%, sodium 0.3%,
potassium 0.3%.
The mantle differs in elemental composition from the crust in
having a great deal more magnesium and significantly more iron, while
having much less aluminum and sodium.
Core
Due to mass segregation,
the core of the Earth is believed to be primarily composed of iron
(88.8%), with smaller amounts of nickel (5.8%), sulfur (4.5%), and less
than 1% trace elements.
Ocean
The most abundant elements in the ocean by proportion of mass in
percent are oxygen (85.84), hydrogen (10.82), chlorine (1.94), sodium
(1.08), magnesium (0.1292), sulfur (0.091), calcium (0.04), potassium
(0.04), bromine (0.0067), carbon (0.0028), and boron (0.00043).
Atmosphere
The order of elements by volume-fraction (which is approximately molecular mole-fraction) in the atmosphere is nitrogen (78.1%), oxygen (20.9%), argon
(0.96%), followed by (in uncertain order) carbon and hydrogen because
water vapor and carbon dioxide, which represent most of these two
elements in the air, are variable components. Sulfur, phosphorus, and
all other elements are present in significantly lower proportions.
According to the abundance curve graph (above right), argon, a
significant if not major component of the atmosphere, does not appear in
the crust at all. This is because the atmosphere has a far smaller mass
than the crust, so argon remaining in the crust contributes little to
mass-fraction there, while at the same time buildup of argon in the
atmosphere has become large enough to be significant.
Human body
By mass, human cells consist of 65–90% water (H2O), and a
significant portion of the remainder is composed of carbon-containing
organic molecules. Oxygen therefore contributes a majority of a human
body's mass, followed by carbon. Almost 99% of the mass of the human
body is made up of six elements: hydrogen (H), carbon (C), nitrogen (N), oxygen (O), calcium (Ca), and phosphorus (P). The next 0.75% is made up of the next five elements: potassium (K), sulfur (S), chlorine (Cl), sodium (Na), and magnesium (Mg). CHNOPS
for short. Only 17 elements are known for certain to be necessary to
human life, with one additional element (fluorine) thought to be helpful
for tooth enamel strength. A few more trace elements may play some role in the health of mammals. Boron and silicon
are notably necessary for plants but have uncertain roles in animals.
The elements aluminium and silicon, although very common in the earth's
crust, are conspicuously rare in the human body.
Below is a periodic table highlighting nutritional elements.
| H | He | |||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | |||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |
| Cs | Ba | La | * | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac | ** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| * | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| ** | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | ||||
Quantity elements
|
Essential trace elements
|
Deemed essential trace element by U.S., not by European Union
|
Suggested
function from deprivation effects or active metabolic handling, but no
clearly-identified biochemical function in humans
|
Limited circumstantial evidence for trace benefits or biological action in mammals
|
No evidence for biological action in mammals, but essential in some lower organisms.
(In the case of lanthanum, the definition of an essential nutrient as being indispensable and irreplaceable is not completely applicable due to the extreme similarity of the lanthanides. Thus Ce, Pr, and Nd may be substituted for La without ill effects for organisms using La, and the smaller Sm, Eu, and Gd may also be similarly substituted but cause slower growth.) |