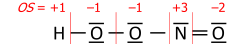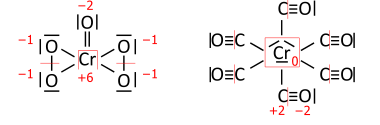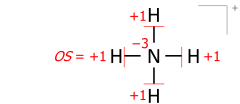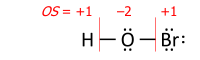Electrostatic
potential map of a water molecule, where the oxygen atom has a more
negative charge (red) than the positive (blue) hydrogen atoms
Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract a shared pair of electrons (or electron density) towards itself. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons
reside from the charged nucleus. The higher the associated
electronegativity number, the more an atom or a substituent group
attracts electrons towards itself.
On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more "pull" it will have on electrons) and the number/location of other electrons present in the atomic shells (the more electrons an atom has, the farther from the nucleus
the valence electrons will be, and as a result the less positive charge
they will experience—both because of their increased distance from the
nucleus, and because the other electrons in the lower energy core orbitals will act to shield the valence electrons from the positively charged nucleus).
The opposite of electronegativity is electropositivity: a measure of an element's ability to donate electrons.
The term "electronegativity" was introduced by Jöns Jacob Berzelius in 1811,
though the concept was known even before that and was studied by many chemists including Avogadro.
In spite of its long history, an accurate scale of electronegativity was not developed until 1932, when Linus Pauling proposed an electronegativity scale, which depends on bond energies, as a development of valence bond theory.
It has been shown to correlate with a number of other chemical
properties. Electronegativity cannot be directly measured and must be
calculated from other atomic or molecular properties. Several methods of
calculation have been proposed, and although there may be small
differences in the numerical values of the electronegativity, all
methods show the same periodic trends between elements.
The most commonly used method of calculation is that originally proposed by Linus Pauling. This gives a dimensionless quantity, commonly referred to as the Pauling scale (χr), on a relative scale running from 0.79 to 3.98 (hydrogen =
2.20). When other methods of calculation are used, it is conventional
(although not obligatory) to quote the results on a scale that covers
the same range of numerical values: this is known as an
electronegativity in Pauling units.
As it is usually calculated, electronegativity is not a property of an atom alone, but rather a property of an atom in a molecule. Properties of a free atom include ionization energy and electron affinity. It is to be expected that the electronegativity of an element will vary with its chemical environment, but it is usually considered to be a transferable property, that is to say that similar values will be valid in a variety of situations.
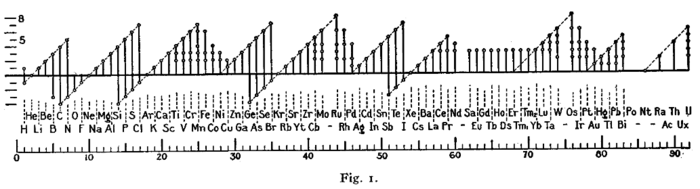
Caesium is the least electronegative element in the periodic table (=0.79), while fluorine is most electronegative (=3.98). Francium
and caesium were originally both assigned 0.7; caesium's value was
later refined to 0.79, but no experimental data allows a similar
refinement for francium. However, francium's ionization energy is known to be slightly higher than caesium's, in accordance with the relativistic stabilization of the 7s orbital, and this in turn implies that francium is in fact more electronegative than caesium.
Electronegativities of the elements
Methods of calculation
Pauling electronegativity
Pauling first proposed the concept of electronegativity in 1932 as an explanation of the fact that the covalent bond between two different atoms (A–B) is stronger than would be expected by taking the average of the strengths of the A–A and B–B bonds. According to valence bond theory, of which Pauling was a notable proponent, this "additional stabilization" of the heteronuclear bond is due to the contribution of ionic canonical forms to the bonding.The difference in electronegativity between atoms A and B is given by:
As only differences in electronegativity are defined, it is necessary to choose an arbitrary reference point in order to construct a scale. Hydrogen was chosen as the reference, as it forms covalent bonds with a large variety of elements: its electronegativity was fixed first at 2.1, later revised to 2.20. It is also necessary to decide which of the two elements is the more electronegative (equivalent to choosing one of the two possible signs for the square root). This is usually done using "chemical intuition": in the above example, hydrogen bromide dissolves in water to form H+ and Br− ions, so it may be assumed that bromine is more electronegative than hydrogen. However, in principle, since the same electronegativities should be obtained for any two bonding compounds, the data are in fact overdetermined, and the signs are unique once a reference point is fixed (usually, for H or F).
To calculate Pauling electronegativity for an element, it is necessary to have data on the dissociation energies of at least two types of covalent bond formed by that element. A. L. Allred updated Pauling's original values in 1961 to take account of the greater availability of thermodynamic data, and it is these "revised Pauling" values of the electronegativity that are most often used.
The essential point of Pauling electronegativity is that there is an underlying, quite accurate, semi-empirical formula for dissociation energies, namely:
The geometric mean is approximately equal to the arithmetic mean - which is applied in the first formula above - when the energies are of the similar value, e.g., except for the highly electropositive elements, where there is a larger difference of two dissociation energies; the geometric mean is more accurate and almost always gives a positive excess energy, due to ionic bonding. The square root of this excess energy, Pauling notes, is approximately additive, and hence one can introduce the electronegativity. Thus, it is this semi-empirical formula for bond energy that underlies Pauling electronegativity concept.
The formulas are approximate, but this rough approximation is in fact relatively good and gives the right intuition, with the notion of polarity of the bond and some theoretical grounding in quantum mechanics. The electronegativities are then determined to best fit the data.
In more complex compounds, there is additional error since electronegativity depends on the molecular environment of an atom. Also, the energy estimate can be only used for single, not for multiple bonds. The energy of formation of a molecule containing only single bonds then can be approximated from an electronegativity table, and depends on the constituents and sum of squares of differences of electronegativities of all pairs of bonded atoms. Such a formula for estimating energy typically has relative error of order of 10%, but can be used to get a rough qualitative idea and understanding of a molecule.
Mulliken electronegativity
The correlation between Mulliken electronegativities (x-axis, in kJ/mol) and Pauling electronegativities (y-axis).
Allred–Rochow electronegativity
The correlation between Allred–Rochow electronegativities (x-axis, in Å−2) and Pauling electronegativities (y-axis).
Sanderson electronegativity equalization
The correlation between Sanderson electronegativities (x-axis, arbitrary units) and Pauling electronegativities (y-axis).
Allen electronegativity
The correlation between Allen electronegativities (x-axis, in kJ/mol) and Pauling electronegativities (y-axis).
The one-electron energies can be determined directly from spectroscopic data, and so electronegativities calculated by this method are sometimes referred to as spectroscopic electronegativities. The necessary data are available for almost all elements, and this method allows the estimation of electronegativities for elements that cannot be treated by the other methods, e.g. francium, which has an Allen electronegativity of 0.67. However, it is not clear what should be considered to be valence electrons for the d- and f-block elements, which leads to an ambiguity for their electronegativities calculated by the Allen method.
Correlation of electronegativity with other properties
The variation of the isomer shift (y-axis, in mm/s) of [SnX6]2− anions, as measured by 119Sn Mössbauer spectroscopy, against the sum of the Pauling electronegativities of the halide substituents (x-axis).
Several correlations have been shown between infrared stretching frequencies of certain bonds and the electronegativities of the atoms involved: however, this is not surprising as such stretching frequencies depend in part on bond strength, which enters into the calculation of Pauling electronegativities. More convincing are the correlations between electronegativity and chemical shifts in NMR spectroscopy or isomer shifts in Mössbauer spectroscopy. Both these measurements depend on the s-electron density at the nucleus, and so are a good indication that the different measures of electronegativity really are describing "the ability of an atom in a molecule to attract electrons to itself".
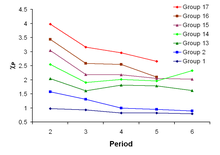
Trends in electronegativity
Periodic trends
The variation of Pauling electronegativity (y-axis) as one descends the main groups of the periodic table from the second period to the sixth period
There are some exceptions to this general rule. Gallium and germanium have higher electronegativities than aluminium and silicon, respectively, because of the d-block contraction. Elements of the fourth period immediately after the first row of the transition metals have unusually small atomic radii because the 3d-electrons are not effective at shielding the increased nuclear charge, and smaller atomic size correlates with higher electronegativity. The anomalously high electronegativity of lead, in particular when compared to thallium and bismuth, appears to be an artifact of data selection (and data availability)—methods of calculation other than the Pauling method show the normal periodic trends for these elements.
Variation of electronegativity with oxidation number
In inorganic chemistry it is common to consider a single value of the electronegativity to be valid for most "normal" situations. While this approach has the advantage of simplicity, it is clear that the electronegativity of an element is not an invariable atomic property and, in particular, increases with the oxidation state of the element.Allred used the Pauling method to calculate separate electronegativities for different oxidation states of the handful of elements (including tin and lead) for which sufficient data was available. However, for most elements, there are not enough different covalent compounds for which bond dissociation energies are known to make this approach feasible. This is particularly true of the transition elements, where quoted electronegativity values are usually, of necessity, averages over several different oxidation states and where trends in electronegativity are harder to see as a result.
| Acid | Formula | Chlorine oxidation state |
pKa |
|---|---|---|---|
| Hypochlorous acid | HClO | +1 | +7.5 |
| Chlorous acid | HClO2 | +3 | +2.0 |
| Chloric acid | HClO3 | +5 | –1.0 |
| Perchloric acid | HClO4 | +7 | –10 |
The chemical effects of this increase in electronegativity can be seen both in the structures of oxides and halides and in the acidity of oxides and oxoacids. Hence CrO3 and Mn2O7 are acidic oxides with low melting points, while Cr2O3 is amphoteric and Mn2O3 is a completely basic oxide.
The effect can also be clearly seen in the dissociation constants of the oxoacids of chlorine. The effect is much larger than could be explained by the negative charge being shared among a larger number of oxygen atoms, which would lead to a difference in pKa of log10(1⁄4) = –0.6 between hypochlorous acid and perchloric acid. As the oxidation state of the central chlorine atom increases, more electron density is drawn from the oxygen atoms onto the chlorine, reducing the partial negative charge on the oxygen atoms and increasing the acidity.
Electronegativity and hybridization scheme
The electronegativity of an atom changes depending on the hybridization of the orbital employed in bonding. Electrons in s orbitals are held more tightly than electrons in p orbitals. Hence, a bond to an atom that employs an spx hybrid orbital for bonding will be more heavily polarized to that atom when the hybrid orbital has more s character. That is, when electronegativities are compared for different hybridization schemes of a given element, the order χ(sp3) < χ(sp2) < χ(sp) holds (the trend should apply to non-integer hybridization indices as well). While this holds true in principle for any main-group element, values for the hybridization-specific electronegativity are most frequently cited for carbon. In organic chemistry, these electronegativities are frequently invoked to predict or rationalize bond polarities in organic compounds containing double and triple bonds to carbon.| Hybridization | χ (Pauling) |
|---|---|
| C(sp3) | 2.3 |
| C(sp2) | 2.6 |
| C(sp) | 3.1 |
| 'generic' C | 2.5 |
Group electronegativity
In organic chemistry, electronegativity is associated more with different functional groups than with individual atoms. The terms group electronegativity and substituent electronegativity are used synonymously. However, it is common to distinguish between the inductive effect and the resonance effect, which might be described as σ- and π-electronegativities, respectively. There are a number of linear free-energy relationships that have been used to quantify these effects, of which the Hammett equation is the best known. Kabachnik parameters are group electronegativities for use in organophosphorus chemistry.Electropositivity
Electropositivity is a measure of an element's ability to donate electrons, and therefore form positive ions; thus, it is opposed to electronegativity.Mainly, this is an attribute of metals, meaning that, in general, the greater the metallic character of an element the greater the electropositivity. Therefore, the alkali metals are most electropositive of all. This is because they have a single electron in their outer shell and, as this is relatively far from the nucleus of the atom, it is easily lost; in other words, these metals have low ionization energies.
While electronegativity increases along periods in the periodic table, and decreases down groups, electropositivity decreases along periods (from left to right) and increases down groups.