Epigenetics patterns in a normal and cancer cells
Epigenetic alterations in tumour progression
Mechanisms
DNA methylation
A DNA molecule fragment that is methylated at two cytosines
In somatic cells, patterns of DNA methylation are in general transmitted to daughter cells with high fidelity. However, epigenetic DNA methylation differs between normal cells and tumor cells in humans. The "normal" CpG methylation profile is often inverted in cells that become tumorigenic. In normal cells, CpG islands preceding gene promoters are generally unmethylated, and tend to be transcriptionally active, while other individual CpG dinucleotides throughout the genome tend to be methylated. However, in cancer cells, CpG islands preceding tumor suppressor gene promoters are often hypermethylated, while CpG methylation of oncogene promoter regions and parasitic repeat sequences is often decreased.
Hypermethylation of tumor suppressor gene promoter regions can
result in silencing of those genes. This type of epigenetic mutation
allows cells to grow and reproduce uncontrollably, leading to
tumorigenesis.
Genes commonly found to be transcriptionally silenced due to promoter
hypermethylation include: Cyclin-dependent kinase inhibitor p16, a cell-cycle inhibitor; MGMT, a DNA repair gene; APC, a cell cycle regulator; MLH1, a DNA-repair gene; and BRCA1, another DNA-repair gene.
Indeed, cancer cells can become addicted to the transcriptional
silencing, due to promoter hypermethylation, of some key tumor
suppressor genes, a process known as epigenetic addiction.
Hypomethylation of CpG dinucleotides in other parts of the genome leads to chromosome instability due to mechanisms such as loss of imprinting and reactivation of transposable elements. Loss of imprinting of insulin-like growth factor gene (IGF2) increases risk of colorectal cancer and is associated with Beckwith-Wiedemann syndrome which significantly increases the risk of cancer for newborns. In healthy cells, CpG dinucleotides of lower densities are found within coding and non-coding intergenic regions. Expression of some repetitive sequences and meiotic recombination at centromeres are repressed through methylation.
The entire genome of a cancerous cell contains significantly less methylcytosine
than the genome of a healthy cell. In fact, cancer cell genomes have
20-50% less methylation at individual CpG dinucleotides across the
genome.
CpG islands found in promoter regions are usually protected from DNA
methylation. In cancer cells CpG islands are hypomethylated
The regions flanking CpG islands called CpG island shores are where
most DNA methylation occurs in the CpG dinucleotide context. Cancer
cells are deferentially methylated at CpG island shores. In cancer
cells, hypermethylation in the CpG island shores move into CpG islands,
or hypomethylation of CpG islands move into CpG island shores
eliminating sharp epigenetic boundaries between these genetic elements. In cancer cells "global hypomethylation" due to disruption in DNA methyltransferases (DNMTs) may promote mitotic recombination and chromosome rearrangement, ultimately resulting in aneuploidy when the chromosomes fail to separate properly during mitosis.
CpG island methylation is important in regulation of gene
expression, yet cytosine methylation can lead directly to destabilizing
genetic mutations and a precancerous cellular state. Methylated
cytosines make hydrolysis of the amine group and spontaneous conversion to thymine more favorable. They can cause aberrant recruitment of chromatin proteins. Cytosine methylations change the amount of UV light absorption of the nucleotide base, creating pyrimidine dimers. When mutation results in loss of heterozygosity
at tumor suppressor gene sites, these genes may become inactive. Single
base pair mutations during replication can also have detrimental
effects.
Histone modification
Eukaryotic DNA has a complex structure. It is generally wrapped around special proteins called histones to form a structure called a nucleosome. A nucleosome consists of 2 sets of 4 histones: H2A, H2B, H3, and H4. Additionally, histone H1 contributes to DNA packaging
outside of the nucleosome. Certain histone modifying enzymes can add or
remove functional groups to the histones, and these modifications
influence the level of transcription of the genes wrapped around those
histones and the level of DNA replication. Histone modification profiles
of healthy and cancerous cells tend to differ.
In comparison to healthy cells, cancerous cells exhibit decreased monoacetylated and trimethylated forms of histone H4 (decreased H4ac and H4me3). Additionally, mouse models have shown that a decrease in histone H4R3 asymmetric dimethylation (H4R3me2a) of the p19ARF promoter is correlated with more advanced cases of tumorigenesis and metastasis. In mouse models, the loss of histone H4 acetylation and trimethylation increases as tumor growth continues. Loss of histone H4 Lysine 16 acetylation (H4K16ac), which is a mark of aging at the telomeres, specifically loses its acetylation. Some scientists hope this particular loss of histone acetylation might be battled with a histone deacetylase (HDAC) inhibitor specific for SIRT1, an HDAC specific for H4K16.
Other histone marks associated with tumorigenesis
include increased deacetylation (decreased acetylation) of histones H3
and H4, decreased trimethylation of histone H3 Lysine 4 (H3K4me3), and increased monomethylation of histone H3 Lysine 9 (H3K9me) and trimethylation of histone H3 Lysine 27 (H3K27me3).
These histone modifications can silence tumor suppressor genes despite
the drop in methylation of the gene's CpG island (an event that normally
activates genes).
Some research has focused on blocking the action of BRD4 on acetylated histones, which has been shown to increase the expression of the Myc
protein, implicated in several cancers. The development process of the
drug to bind to BRD4 is noteworthy for the collaborative, open approach
the team is taking.
The tumor suppressor gene p53 regulates DNA repair and can induce apoptosis in dysregulated cells. E Soto-Reyes and F Recillas-Targa elucidated the importance of the CTCF protein in regulating p53 expression.[26] CTCF, or CCCTC binding factor, is a zinc finger protein that insulates the p53 promoter
from accumulating repressive histone marks. In certain types of cancer
cells, the CTCF protein does not bind normally, and the p53 promoter
accumulates repressive histone marks, causing p53 expression to
decrease.
Mutations in the epigenetic machinery itself may occur as well,
potentially responsible for the changing epigenetic profiles of
cancerous cells. The histone
variants of the H2A family are highly conserved in mammals, playing
critical roles in regulating many nuclear processes by altering chromatin
structure. One of the key H2A variants, H2A.X, marks DNA damage,
facilitating the recruitment of DNA repair proteins to restore genomic
integrity. Another variant, H2A.Z, plays an important role in both gene
activation and repression. A high level of H2A.Z expression is detected
in many cancers and is significantly associated with cellular
proliferation and genomic instability. Histone variant macroH2A1 is important in the pathogenesis of many types of cancers, for instance in hepatocellular carcinoma. Other mechanisms include a decrease in H4K16ac may be caused by either a decrease in activity of a histone acetyltransferases (HATs) or an increase in deacetylation by SIRT1. Likewise, an inactivating frameshift mutation in HDAC2, a histone deacetylase that acts on many histone-tail lysines, has been associated with cancers showing altered histone acetylation patterns. These findings indicate a promising mechanism for altering epigenetic profiles through enzymatic inhibition or enhancement.
DNA damage, caused by UV light, ionizing radiation,
environmental toxins, and metabolic chemicals, can also lead to genomic
instability and cancer. The DNA damage response to double strand DNA breaks (DSB) is mediated in part by histone modifications. At a DSB, MRE11-RAD50-NBS1 (MRN) protein complex recruits ataxia telangiectasia
mutated (ATM) kinase which phosphorylates Serine 129 of Histone 2A.
MDC1, mediator of DNA damage checkpoint 1, binds to the phosphopeptide,
and phosphorylation of H2AX may spread by a positive feedback loop of MRN-ATM recruitment and phosphorylation. TIP60 acetylates the γH2AX, which is then polyubiquitylated. RAP80, a subunit of the DNA repair breast cancer type 1 susceptibility protein complex (BRCA1-A), binds ubiquitin attached to histones. BRCA1-A activity arrests the cell cycle at the G2/M checkpoint, allowing time for DNA repair, or apoptosis may be initiated.
MicroRNA gene silencing
In mammals, microRNAs (miRNAs) regulate about 60% of the transcriptional activity of protein-encoding genes. Some miRNAs also undergo methylation-associated silencing in cancer cells. Let-7 and miR15/16 play important roles in down-regulating RAS and BCL2 oncogenes, and their silencing occurs in cancer cells. Decreased expression of miR-125b1, a miRNA that functions as a tumor suppressor, was observed in prostate, ovarian, breast and glial cell cancers. In vitro experiments have shown that miR-125b1 targets two genes, HER2/neu and ESR1,
that are linked to breast cancer. DNA methylation, specifically
hypermethylation, is one of the main ways that the miR-125b1 is
epigenetically silenced. In patients with breast cancer,
hypermethylation of CpG islands located proximal to the transcription
start site was observed. Loss of CTCF binding and an increase in repressive histone marks, H3K9me3 and H3K27me3, correlates with DNA methylation
and miR-125b1 silencing. Mechanistically, CTCF may function as a
boundary element to stop the spread of DNA methylation. Results from
experiments conducted by Soto-Reyes et al.
indicate a negative effect of methylation on the function and
expression of miR-125b1. Therefore, they concluded that DNA methylation
has a part in silencing the gene. Furthermore, some miRNA's are
epigenetically silenced early on in breast cancer, and therefore these
miRNA's could potentially be useful as tumor markers.
The epigenetic silencing of miRNA genes by aberrant DNA methylation is a
frequent event in cancer cells; almost one third of miRNA promoters
active in normal mammary cells were found hypermethylated in breast
cancer cells - that is a several fold greater proportion than is usually
observed for protein coding genes.
Metabolic recoding of epigenetics in cancer
Dysregulation
of metabolism allows tumor cells to generate needed building blocks as
well as to modulate epigenetic marks to support cancer initiation and
progression. Cancer-induced metabolic changes alter the epigenetic
landscape, especially modifications on histones and DNA, thereby
promoting malignant transformation, adaptation to inadequate nutrition,
and metastasis. The accumulation of certain metabolites in cancer can
target epigenetic enzymes to globally alter the epigenetic landscape.
Cancer-related metabolic changes lead to locus-specific recoding of
epigenetic marks. Cancer epigenetics can be precisely reprogramed by
cellular metabolism through 1) dose-responsive modulation of cancer
epigenetics by metabolites; 2) sequence-specific recruitment of
metabolic enzymes; and 3) targeting of epigenetic enzymes by nutritional
signals.
MicroRNA and DNA repair
DNA damage appears to be the primary underlying cause of cancer. If DNA repair is deficient, DNA damage tends to accumulate. Such excess DNA damage can increase mutational errors during DNA replication due to error-prone translesion synthesis. Excess DNA damage can also increase epigenetic alterations due to errors during DNA repair. Such mutations and epigenetic alterations can give rise to cancer.
Germ line mutations in DNA repair genes cause only 2–5% of colon cancer cases.
However, altered expression of microRNAs, causing DNA repair
deficiencies, are frequently associated with cancers and may be an
important causal factor for these cancers.
Over-expression of certain miRNAs may directly reduce expression of specific DNA repair proteins. Wan et al. referred to 6 DNA repair genes that are directly targeted by the miRNAs indicated in parentheses: ATM (miR-421), RAD52 (miR-210, miR-373), RAD23B (miR-373), MSH2 (miR-21), BRCA1 (miR-182) and P53 (miR-504, miR-125b). More recently, Tessitore et al. listed further DNA repair genes that are directly targeted by additional miRNAs, including ATM (miR-18a, miR-101), DNA-PK (miR-101), ATR (miR-185), Wip1 (miR-16), MLH1, MSH2 and MSH6 (miR-155), ERCC3 and ERCC4 (miR-192) and UNG2
(mir-16, miR-34c and miR-199a). Of these miRNAs, miR-16, miR-18a,
miR-21, miR-34c, miR-125b, miR-101, miR-155, miR-182, miR-185 and
miR-192 are among those identified by Schnekenburger and Diederich
as over-expressed in colon cancer through epigenetic hypomethylation.
Over expression of any one of these miRNAs can cause reduced expression
of its target DNA repair gene.
Up to 15% of the MLH1-deficiencies in sporadic colon cancers appeared to be due to over-expression of the microRNA miR-155, which represses MLH1 expression. However, the majority of 68 sporadic colon cancers with reduced expression of the DNA mismatch repair protein MLH1 were found to be deficient due to epigenetic methylation of the CpG island of the MLH1 gene.
In 28% of glioblastomas, the MGMT DNA repair protein is deficient but the MGMT promoter is not methylated. In the glioblastomas without methylated MGMT promoters, the level of microRNA miR-181d is inversely correlated with protein expression of MGMT and the direct target of miR-181d is the MGMT mRNA 3’UTR (the three prime untranslated region of MGMT mRNA).
Thus, in 28% of glioblastomas, increased expression of miR-181d and
reduced expression of DNA repair enzyme MGMT may be a causal factor. In
29–66% of glioblastomas, DNA repair is deficient due to epigenetic methylation of the MGMT gene, which reduces protein expression of MGMT.
High mobility group A (HMGA) proteins, characterized by an AT-hook, are small, nonhistone, chromatin-associated proteins that can modulate transcription. MicroRNAs control the expression of HMGA proteins, and these proteins (HMGA1 and HMGA2) are architectural chromatin transcription-controlling elements. Palmieri et al showed that, in normal tissues, HGMA1 and HMGA2 genes are targeted (and thus strongly reduced in expression) by miR-15, miR-16, miR-26a, miR-196a2 and Let-7a.
HMGA expression is almost undetectable in differentiated adult
tissues but is elevated in many cancers. HGMA proteins are polypeptides
of ~100 amino acid residues characterized by a modular sequence
organization. These proteins have three highly positively charged
regions, termed AT hooks,
that bind the minor groove of AT-rich DNA stretches in specific regions
of DNA. Human neoplasias, including thyroid, prostatic, cervical,
colorectal, pancreatic and ovarian carcinoma, show a strong increase of
HMGA1a and HMGA1b proteins.
Transgenic mice with HMGA1 targeted to lymphoid cells develop
aggressive lymphoma, showing that high HMGA1 expression is not only
associated with cancers, but that the HMGA1 gene can act as an oncogene to cause cancer. Baldassarre et al., showed that HMGA1 protein binds to the promoter region of DNA repair gene BRCA1 and inhibits BRCA1 promoter activity. They also showed that while only 11% of breast tumors had hypermethylation of the BRCA1
gene, 82% of aggressive breast cancers have low BRCA1 protein
expression, and most of these reductions were due to chromatin
remodeling by high levels of HMGA1 protein.
HMGA2 protein specifically targets the promoter of ERCC1, thus reducing expression of this DNA repair gene.
ERCC1 protein expression was deficient in 100% of 47 evaluated colon
cancers (though the extent to which HGMA2 was involved is unknown).
Palmieri et al. showed that each of the miRNAs that target HMGA
genes are drastically reduced in almost all human pituitary adenomas
studied, when compared with the normal pituitary gland. Consistent with
the down-regulation of these HMGA-targeting miRNAs, an increase in the
HMGA1 and HMGA2-specific mRNAs was observed. Three of these microRNAs
(miR-16, miR-196a and Let-7a)
have methylated promoters and therefore low expression in colon cancer.
For two of these, miR-15 and miR-16, the coding regions are
epigenetically silenced in cancer due to histone deacetylase activity.
When these microRNAs are expressed at a low level, then HMGA1 and HMGA2
proteins are expressed at a high level. HMGA1 and HMGA2 target (reduce
expression of) BRCA1 and ERCC1 DNA repair genes. Thus DNA repair can be reduced, likely contributing to cancer progression.
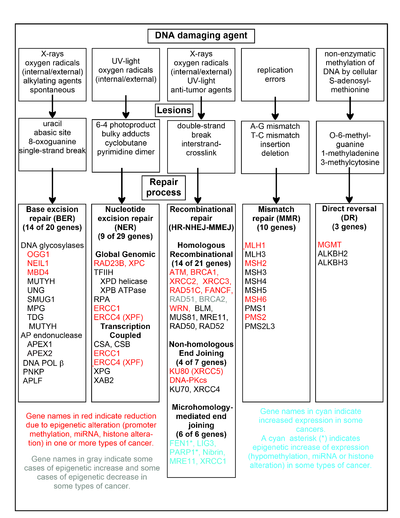
DNA repair pathways
A
chart of common DNA damaging agents, examples of lesions they cause in
DNA, and pathways used to repair these lesions. Also shown are many of
the genes in these pathways, an indication of which genes are
epigenetically regulated to have reduced (or increased) expression in
various cancers. It also shows genes in the error prone
microhomology-mediated end joining pathway with increased expression in
various cancers.
The chart in this section shows some frequent DNA damaging agents,
examples of DNA lesions they cause, and the pathways that deal with
these DNA damages. At least 169 enzymes are either directly employed in
DNA repair or influence DNA repair processes. Of these, 83 are directly employed in repairing the 5 types of DNA damages illustrated in the chart.
Some of the more well studied genes central to these repair
processes are shown in the chart. The gene designations shown in red,
gray or cyan indicate genes frequently epigenetically altered in various
types of cancers. Wikipedia articles on each of the genes highlighted
by red, gray or cyan describe the epigenetic alteration(s) and the
cancer(s) in which these epimutations are found. Three review articles, and two broad experimental survey articles also document most of these epigenetic DNA repair deficiencies in cancers.
Red-highlighted genes are frequently reduced or silenced by
epigenetic mechanisms in various cancers. When these genes have low or
absent expression, DNA damages can accumulate. Replication errors past
these damages can lead to increased mutations and, ultimately, cancer. Epigenetic repression of DNA repair genes in accurate DNA repair pathways appear to be central to carcinogenesis.
The two gray-highlighted genes RAD51 and BRCA2, are required for homologous recombinational
repair. They are sometimes epigenetically over-expressed and sometimes
under-expressed in certain cancers. As indicated in the Wikipedia
articles on RAD51 and BRCA2,
such cancers ordinarily have epigenetic deficiencies in other DNA
repair genes. These repair deficiencies would likely cause increased
unrepaired DNA damages. The over-expression of RAD51 and BRCA2 seen in these cancers may reflect selective pressures for compensatory RAD51 or BRCA2
over-expression and increased homologous recombinational repair to at
least partially deal with such excess DNA damages. In those cases where RAD51 or BRCA2 are under-expressed, this would itself lead to increased unrepaired DNA damages. Replication errors past these damages could cause increased mutations and cancer, so that under-expression of RAD51 or BRCA2 would be carcinogenic in itself.
Cyan-highlighted genes are in the microhomology-mediated end joining (MMEJ) pathway and are up-regulated in cancer. MMEJ is an additional error-prone inaccurate
repair pathway for double-strand breaks. In MMEJ repair of a
double-strand break, an homology of 5-25 complementary base pairs
between both paired strands is sufficient to align the strands, but
mismatched ends (flaps) are usually present. MMEJ removes the extra
nucleotides (flaps) where strands are joined, and then ligates the
strands to create an intact DNA double helix. MMEJ almost always
involves at least a small deletion, so that it is a mutagenic pathway. FEN1,
the flap endonuclease in MMEJ, is epigenetically increased by promoter
hypomethylation and is over-expressed in the majority of cancers of the
breast, prostate, stomach, neuroblastomas, pancreas, and lung. PARP1 is also over-expressed when its promoter region ETS site is epigenetically hypomethylated, and this contributes to progression to endometrial cancer, BRCA-mutated ovarian cancer, and BRCA-mutated serous ovarian cancer. Other genes in the MMEJ pathway are also over-expressed in a number of cancers (see MMEJ for summary), and are also shown in blue.
Frequencies of epimutations in DNA repair genes
Deficiencies
in DNA repair proteins that function in accurate DNA repair pathways
increase the risk of mutation. Mutation rates are strongly increased in
cells with mutations in DNA mismatch repair or in homologous recombinational repair (HRR). Individuals with inherited mutations in any of 34 DNA repair genes are at increased risk of cancer.
In sporadic cancers, a deficiency in DNA repair is occasionally
found to be due to a mutation in a DNA repair gene, but much more
frequently reduced or absent expression of DNA repair genes is due to
epigenetic alterations that reduce or silence gene expression. For
example, for 113 colorectal cancers examined in sequence, only four had a
missense mutation in the DNA repair gene MGMT, while the majority had reduced MGMT expression due to methylation of the MGMT promoter region (an epigenetic alteration). Similarly, out of 119 cases of mismatch repair-deficient colorectal cancers that lacked DNA repair gene PMS2 expression, PMS2 protein was deficient in 6 due to mutations in the PMS2
gene, while in 103 cases PMS2 expression was deficient because its
pairing partner MLH1 was repressed due to promoter methylation (PMS2
protein is unstable in the absence of MLH1).
In the other 10 cases, loss of PMS2 expression was likely due to
epigenetic overexpression of the microRNA, miR-155, which down-regulates
MLH1.
Epigenetic defects in DNA repair genes are frequent in cancers.
In the table, multiple cancers were evaluated for reduced or absent
expression of the DNA repair gene of interest, and the frequency shown
is the frequency with which the cancers had an epigenetic deficiency of
gene expression. Such epigenetic deficiencies likely arise early in carcinogenesis, since they are also frequently found (though at somewhat lower frequency) in the field defect surrounding the cancer from which the cancer likely arose (see Table).
| Cancer | Gene | Frequency in Cancer | Frequency in Field Defect |
|---|---|---|---|
| Colorectal | MGMT | 46% | 34% |
| Colorectal | MGMT | 47% | 11% |
| Colorectal | MGMT | 70% | 60% |
| Colorectal | MSH2 | 13% | 5% |
| Colorectal | ERCC1 | 100% | 40% |
| Colorectal | PMS2 | 88% | 50% |
| Colorectal | XPF | 55% | 40% |
| Head and Neck | MGMT | 54% | 38% |
| Head and Neck | MLH1 | 33% | 25% |
| Head and Neck | MLH1 | 31% | 20% |
| Stomach | MGMT | 88% | 78% |
| Stomach | MLH1 | 73% | 20% |
| Esophagus | MLH1 | 77%–100% | 23%–79% |
It appears that cancers may frequently be initiated by an epigenetic
reduction in expression of one or more DNA repair enzymes. Reduced DNA
repair likely allows accumulation of DNA damages. Error prone translesion synthesis
past some of these DNA damages may give rise to a mutation with a
selective advantage. A clonal patch with a selective advantage may grow
and out-compete neighboring cells, forming a field defect. While there is no obvious selective advantage for a cell to have reduced DNA repair, the epimutation
of the DNA repair gene may be carried along as a passenger when the
cells with the selectively advantageous mutation are replicated. In the
cells carrying both the epimutation of the DNA repair gene and the
mutation with the selective advantage, further DNA damages will
accumulate, and these could, in turn, give rise to further mutations
with still greater selective advantages. Epigenetic defects in DNA
repair may thus contribute to the characteristic high frequency of
mutations in the genomes of cancers, and cause their carcinogenic
progression.
Cancers have high levels of genome instability, associated with a high frequency of mutations.
A high frequency of genomic mutations increases the likelihood of
particular mutations occurring that activate oncogenes and inactivate
tumor suppressor genes, leading to carcinogenesis. On the basis of whole genome sequencing, cancers are found to have thousands to hundreds of thousands of mutations in their whole genomes.
By comparison, the mutation frequency in the whole genome between
generations for humans (parent to child) is about 70 new mutations per
generation.
In the protein coding regions of the genome, there are only about 0.35
mutations between parent/child generations (less than one mutated
protein per generation).
Whole genome sequencing in blood cells for a pair of identical twin
100-year-old centenarians only found 8 somatic differences, though
somatic variation occurring in less than 20% of blood cells would be
undetected.
While DNA damages may give rise to mutations through error prone translesion synthesis, DNA damages can also give rise to epigenetic alterations during faulty DNA repair processes.
The DNA damages that accumulate due to epigenetic DNA repair defects
can be a source of the increased epigenetic alterations found in many
genes in cancers. In an early study, looking at a limited set of
transcriptional promoters, Fernandez et al.
examined the DNA methylation profiles of 855 primary tumors. Comparing
each tumor type with its corresponding normal tissue, 729 CpG island
sites (55% of the 1322 CpG sites evaluated) showed differential DNA
methylation. Of these sites, 496 were hypermethylated (repressed) and
233 were hypomethylated (activated). Thus, there is a high level of
epigenetic promoter methylation alterations in tumors. Some of these
epigenetic alterations may contribute to cancer progression.
Epigenetic carcinogens
A variety of compounds are considered as epigenetic carcinogens—they result in an increased incidence of tumors, but they do not show mutagen
activity (toxic compounds or pathogens that cause tumors incident to
increased regeneration should also be excluded). Examples include diethylstilbestrol, arsenite, hexachlorobenzene, and nickel compounds.
Many teratogens exert specific effects on the fetus by epigenetic mechanisms. While epigenetic effects may preserve the effect of a teratogen such as diethylstilbestrol
throughout the life of an affected child, the possibility of birth
defects resulting from exposure of fathers or in second and succeeding
generations of offspring has generally been rejected on theoretical
grounds and for lack of evidence. However, a range of male-mediated abnormalities have been demonstrated, and more are likely to exist. FDA label information for Vidaza, a formulation of 5-azacitidine
(an unmethylatable analog of cytidine that causes hypomethylation when
incorporated into DNA) states that "men should be advised not to father a
child" while using the drug, citing evidence in treated male mice of
reduced fertility, increased embryo loss, and abnormal embryo
development. In rats, endocrine differences were observed in offspring of males exposed to morphine. In mice, second generation effects of diethylstilbesterol have been described occurring by epigenetic mechanisms.
Cancer subtypes
Skin cancer
Melanoma
is a deadly skin cancer that originates from melanocytes. Several
epigenetic alterations are known to play a role in the transition of
melanocytes to melanoma cells. These alterations are the consequence of
deregulation of their corresponding enzymes. Several histone
methyltransferases and demethylases are among these enzymes.
Prostate cancer
Prostate cancer kills around 35,000 men yearly, and about 220,000 men are diagnosed with prostate cancer per year, in North America alone.
Prostate cancer is the second leading cause of cancer-caused fatalities
in men, and within a man's lifetime, one in six men will have the
disease. Alterations in histone acetylation and DNA methylation occur in various genes influencing prostate cancer. More than 90% of prostate cancers show gene silencing by CpG island hypermethylation of the GSTP1 gene promoter, which protects prostate cells from genomic damage that is caused by different oxidants or carcinogens. Real-time methylation-specific polymerase chain reaction (PCR) suggests that many other genes are also hypermethylated. Gene expression in the prostate may be modulated by nutrition and lifestyle changes.
Cervical cancer
The second most common malignant tumor in women is invasive cervical cancer (ICC) and more than 50% of all invasive cervical cancer (ICC) is caused by oncongenic human papillomavirus 16 (HPV16). Furthermore, cervix intraepithelial neoplasia (CIN) is primarily caused by oncogenic HPV16.
As in many cases, the causative factor for cancer does not always take a
direct route from infection to the development of cancer. Genomic
methylation patterns have been associated with invasive cervical cancer.
Within the HPV16L1 region, 14 tested CpG sites have significantly higher methylation in CIN3+ than in HPV16 genomes of women without CIN3.
Only 2/16 CpG sites tested in HPV16 upstream regulatory region were
found to have association with increased methylation in CIN3+.
This suggests that the direct route from infection to cancer is
sometimes detoured to a precancerous state in cervix intraepithelial
neoplasia. Additionally, increased CpG site methylation was found in low
levels in most of the five host nuclear genes studied, including 5/5 TERT, 1/4 DAPK1, 2/5 RARB, MAL, and CADM1. Furthermore, 1/3 of CpG sites in mitochondrial DNA were associated with increased methylation in CIN3+. Thus, a correlation exists between CIN3+ and increased methylation of CpG sites in the HPV16 L1 open reading frame. This could be a potential biomarker for future screens of cancerous and precancerous cervical disease.
Leukemia
Recent studies have shown that the mixed-lineage leukemia (MLL) gene causes leukemia by rearranging and fusing with other genes in different chromosomes, which is a process under epigenetic control.
Sarcoma
There
are about 15,000 new cases of sarcoma in the US each year, and about
6,200 people were projected to die of sarcoma in the US in 2014.
Sarcomas comprise a large number of rare, histogenetically
heterogeneous mesenchymal tumors that, for example, include
chondrosarcoma, Ewing's sarcoma, leiomyosarcoma, liposarcoma,
osteosarcoma, synovial sarcoma, and (alveolar and embryonal)
rhabdomyosarcoma. Several oncogenes and tumor suppressor genes are
epigenetically altered in sarcomas. These include APC, CDKN1A, CDKN2A,
CDKN2B, Ezrin, FGFR1, GADD45A, MGMT, STK3, STK4, PTEN, RASSF1A, WIF1, as
well as several miRNAs.
Expression of epigenetic modifiers such as that of the BMI1 component
of the PRC1 complex is deregulated in chondrosarcoma, Ewing's sarcoma,
and osteosarcoma, and expression of the EZH2 component of the PRC2
complex is altered in Ewing's sarcoma and rhabdomyosarcoma. Similarly,
expression of another epigenetic modifier, the LSD1 histone demethylase,
is increased in chondrosarcoma, Ewing's sarcoma, osteosarcoma, and
rhabdomyosarcoma. Drug targeting and inhibition of EZH2 in Ewing's
sarcoma, or of LSD1 in several sarcomas, inhibits tumor cell growth in these sarcomas.
Identification methods
Previously,
epigenetic profiles were limited to individual genes under scrutiny by a
particular research team. Recently, however, scientists have been
moving toward a more genomic approach to determine an entire genomic
profile for cancerous versus healthy cells.
Popular approaches for measuring CpG methylation in cells include:
- Bisulfite sequencing
- Combined bisulfite restriction analysis (COBRA)
- Methylation-specific PCR
- MethyLight
- Pyrosequencing
- Restriction landmark genomic scanning
- Arbitrary primed PCR
- HELP assay (HpaII tiny fragment enrichment by ligation-mediated PCR)
- Chromatin immunoprecipitation ChIP-Chip using antibodies specific for methyl-CpG binding domain proteins
- Methylated DNA immunoprecipitation Methyl-DIP
- Gene-expression profiles via DNA microarray : comparing mRNA levels from cancer cell lines before and after treatment with a demethylating agent
Since bisulfite sequencing is considered the gold standard for
measuring CpG methylation, when one of the other methods is used,
results are usually confirmed using bisulfite sequencing.
Popular approaches for determining histone modification profiles in cancerous versus healthy cells include:
- Mass spectrometry
- Chromatin Immunoprecipitation Assay
Diagnosis and prognosis
Researchers
are hoping to identify specific epigenetic profiles of various types
and subtypes of cancer with the goal of using these profiles as tools to
diagnose individuals more specifically and accurately.
Since epigenetic profiles change, scientists would like to use the
different epigenomic profiles to determine the stage of development or
level of aggressiveness of a particular cancer in patients. For example,
hypermethylation of the genes coding for Death-Associated Protein Kinase (DAPK), p16, and Epithelial Membrane Protein 3 (EMP3) have been linked to more aggressive forms of lung, colorectal, and brain cancers. This type of knowledge can affect the way that doctors will diagnose and choose to treat their patients.
Another factor that will influence the treatment of patients is
knowing how well they will respond to certain treatments. Personalized
epigenomic profiles of cancerous cells can provide insight into this
field. For example, MGMT is an enzyme that reverses the addition of alkyl groups to the nucleotide guanine. Alkylating guanine, however, is the mechanism by which several chemotherapeutic drugs act in order to disrupt DNA and cause cell death.
Therefore, if the gene encoding MGMT in cancer cells is hypermethylated
and in effect silenced or repressed, the chemotherapeutic drugs that
act by methylating guanine will be more effective than in cancer cells
that have a functional MGMT enzyme.
Epigenetic biomarkers can also be utilized as tools for molecular prognosis. In primary tumor and mediastinal lymph node biopsy samples, hypermethylation of both CDKN2A and CDH13 serves as the marker for increased risk of faster cancer relapse and higher death rate of patients.
Treatment
Epigenetic control of the proto-onco regions and the tumor suppressor
sequences by conformational changes in histones plays a role in the
formation and progression of cancer. Pharmaceuticals that reverse epigenetic changes might have a role in a variety of cancers.
Recently, it is evidently known that associations between
specific cancer histotypes and epigenetic changes can facilitate the
development of novel epi-drugs. Drug development has focused mainly on modifying DNA methyltransferase, histone acetyltransferase (HAT) and histone deacetylase (HDAC).
Drugs that specifically target the inverted methylation pattern of cancerous cells include the DNA methyltransferase inhibitors azacitidine and decitabine. These hypomethylating agents are used to treat myelodysplastic syndrome, a blood cancer produced by abnormal bone marrow stem cells.
These agents inhibit all three types of active DNA methyltransferases,
and had been thought to be highly toxic, but proved to be effective when
used in low dosage, reducing progression of myelodysplastic syndrome to
leukemia.
Histone deacetylase (HDAC) inhibitors show efficacy in treatment of T cell lymphoma. two HDAC inhibitors, vorinostat and romidepsin, have been approved by the Food and Drug Administration. However, since these HDAC inhibitors alter the acetylation
state of many proteins in addition to the histone of interest,
knowledge of the underlying mechanism at the molecular level of patient
response is required to enhance the efficiency of using such inhibitors
as treatment. Treatment with HDAC inhibitors has been found to promote gene reactivation after DNA methyl-transferases inhibitors have repressed transcription. Panobinostat is approved for certain situations in myeloma.
Other pharmaceutical targets in research are histone lysine methyltransferases (KMT) and protein arginine methyltransferases (PRMT). Preclinical study has suggested that lunasin may have potentially beneficial epigenetic effects.