| ||||||
| Identifiers | ||||||
|---|---|---|---|---|---|---|
| Aliases | AR, AIS, AR8, DHTR, HUMARA, HYSP1, KD, NR3C4, SBMA, SMAX1, TFM, androgen receptor | |||||
| External IDs | OMIM: 313700 MGI: 88064 HomoloGene: 28 GeneCards: AR | |||||
|
| Orthologs | ||||||
|---|---|---|---|---|---|---|
| Species | Human | Mouse | ||||
| Entrez | ||||||
| Ensembl |
| |||||
| UniProt | ||||||
| RefSeq (mRNA) |
|
| ||||
| RefSeq (protein) | ||||||
|
| |||
| Location (UCSC) | n/a | Chr X: 98.15 – 98.32 Mb | ||
|---|---|---|---|---|
| PubMed search |
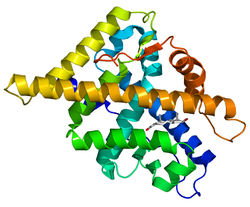
| Androgen_recep | |||
|---|---|---|---|

crystal
structure of the human androgen receptor ligand binding domain bound
with an androgen receptor nh2-terminal peptide, ar20-30, and r1881
| |||
| Identifiers | |||
| Symbol | Androgen_recep | ||
| Pfam | PF02166 | ||
| InterPro | IPR001103 | ||
|
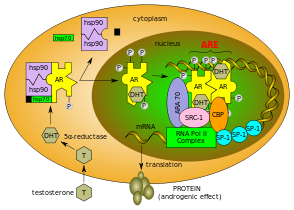
Normal
function of the androgen receptor. Testosterone (T) enters the cell
and, if 5-alpha-reductase is present, is converted into dihydrotestone
(DHT). Upon steroid binding, the androgen receptor (AR) undergoes a
conformational change and releases heat-shock proteins (hsps).
Phosphorylation (P) occurs before or after steroid binding. The AR
translocates to the nucleus where dimerization, DNA binding, and the
recruitment of coactivators occur. Target genes are transcribed (mRNA)
and translated into proteins.
The androgen receptor (AR), also known as NR3C4 (nuclear receptor subfamily 3, group C, member 4), is a type of nuclear receptor that is activated by binding any of the androgenic hormones, including testosterone and dihydrotestosterone in the cytoplasm and then translocating into the nucleus. The androgen receptor is most closely related to the progesterone receptor, and progestins in higher dosages can block the androgen receptor.
The main function of the androgen receptor is as a DNA-binding transcription factor that regulates gene expression; however, the androgen receptor has other functions as well. Androgen regulated genes are critical for the development and maintenance of the male sexual phenotype.
Function
Effect on development
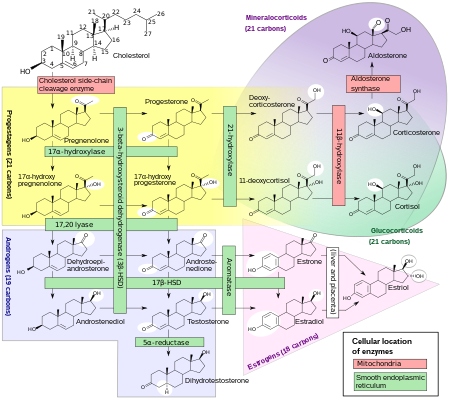
In some cell types, testosterone interacts directly with androgen receptors, whereas, in others, testosterone is converted by 5-alpha-reductase to dihydrotestosterone, an even more potent agonist for androgen receptor activation. Testosterone appears to be the primary androgen receptor-activating hormone in the Wolffian duct, whereas dihydrotestosterone is the main androgenic hormone in the urogenital sinus, urogenital tubercle, and hair follicles. Testosterone is therefore responsible primarily for the development of male primary sexual characteristics, whilst dihydrotestosterone is responsible for secondary male characteristics.
Androgens cause slow epiphysis, or maturation of the bones, but more of the potent epiphysis effect comes from the estrogen produced by aromatization
of androgens. Steroid users of teen age may find that their growth had
been stunted by androgen and/or estrogen excess. People with too little
sex hormones can be short during puberty but end up taller as adults as
in androgen insensitivity syndrome or estrogen insensitivity syndrome.
Also, AR knockout-mice
studies have shown that AR is essential for normal female fertility,
being required for development and full functionality of the ovarian follicles and ovulation, working through both intra-ovarian and neuroendocrine mechanisms.
Maintenance of male skeletal integrity
Via
the androgen receptor, androgens play a key role in the maintenance of
male skeletal integrity. The regulation of this integrity by androgen
receptor (AR) signaling can be attributed to both osteoblasts and
osteocytes.
Mechanism of action
Genomic
The primary mechanism of action for androgen receptors is direct regulation of gene transcription. The binding of an androgen to the androgen receptor results in a conformational change in the receptor that, in turn, causes dissociation of heat shock proteins, transport from the cytosol into the cell nucleus, and dimerization. The androgen receptor dimer binds to a specific sequence of DNA known as a hormone response element. Androgen receptors interact with other proteins in the nucleus, resulting in up- or down-regulation of specific gene transcription. Up-regulation or activation of transcription results in increased synthesis of messenger RNA, which, in turn, is translated by ribosomes
to produce specific proteins. One of the known target genes of androgen
receptor activation is the insulin-like growth factor I receptor (IGF-1R). Thus, changes in levels of specific proteins in cells is one way that androgen receptors control cell behavior.
One function of androgen receptor that is independent of direct
binding to its target DNA sequence, is facilitated by recruitment via
other DNA-binding proteins. One example is serum response factor, a protein that activates several genes that cause muscle growth.
Androgen receptor is modified by post translational modification through acetylation, which directly promotes AR mediated transactivation, apoptosis and contact independent growth of prostate cancer cells. AR acetylation is induced by androgens and determines recruitment into chromatin. The AR acetylation site is a key target of NAD-dependent and TSA-dependent histone deacetylases and long non coding RNA.
Non-genomic
More
recently, androgen receptors have been shown to have a second mode of
action. As has been also found for other steroid hormone receptors such
as estrogen receptors, androgen receptors can have actions that are independent of their interactions with DNA. Androgen receptors interact with certain signal transduction
proteins in the cytoplasm. Androgen binding to cytoplasmic androgen
receptors can cause rapid changes in cell function independent of
changes in gene transcription, such as changes in ion transport.
Regulation of signal transduction pathways by cytoplasmic androgen
receptors can indirectly lead to changes in gene transcription, for
example, by leading to phosphorylation of other transcription factors.
Genetics
Gene
In humans, the androgen receptor is encoded by the AR gene located on the X chromosome at Xq11-12.
AR deficiencies
The androgen insensitivity syndrome,
formerly known as testicular feminization, is caused by a mutation of
the androgen receptor gene located on the X chromosome
(locus:Xq11-Xq12).
The androgen receptor seems to affect neuron physiology and is defective in Kennedy's disease. In addition, point mutations and trinucleotide repeat polymorphisms has been linked to a number of additional disorders.
CAG repeats
The
AR gene contains CAG repeats which affect receptor function, where
fewer repeats leads to increased receptor sensitivity to circulating
androgens and more repeats leads to decreased receptor sensitivity.
Studies have shown that racial variation in CAG repeats exists, with African-Americans having fewer repeats than non-Hispanic white Americans. The racial trends in CAG repeats parallels the incidence and mortality of prostate cancer in these groups.
Structure
Structural
domains of the two isoforms (AR-A and AR-B) of the human androgen
receptor. Numbers above the bars refer to the amino acid residues that
separate the domains starting from the N-terminus (left) to C-terminus
(right). NTD = N-terminal domain, DBD = DNA binding domain. LBD = ligand
binding domain. AF = activation function.
Isoforms
Two isoforms of the androgen receptor (A and B) have been identified:
- AR-A - 87 kDa - N-terminus truncated (lacks the first 187 amino acids), which results from in vitro proteolysis.
- AR-B - 110 kDa - full length
Domains
Like other nuclear receptors, the androgen receptor is modular in structure and is composed of the following functional domains labeled A through F:
- A/B) - N-terminal regulatory domain contains:
- activation function 1 (AF-1) between residues 101 and 370 required for full ligand activated transcriptional activity
- activation function 5 (AF-5) between residues 360-485 is responsible for the constitutive activity (activity without bound ligand)
- dimerization surface involving residues 1-36 (containing the FXXLF motif where F = phenylalanine, L = leucine, and X = any amino acid residue) and 370-494, both of which interact with the LBD in an intramolecular head-to-tail interaction
- C) - DNA binding domain (DBD)
- D) - Hinge region - flexible region that connects the DBD with the LBD; along with the DBD, contains a ligand dependent nuclear localization signal
- E) - Ligand binding domain (LBD) containing
- activation function 2 (AF-2), responsible for agonist induced activity (activity in the presence of bound agonist)
- AF-2 binds either the N-terminal FXXFL motif intramolecularly or coactivator proteins (containing the LXXLL or preferably FXXFL motifs)
- A ligand dependent nuclear export signal
- F) - C-terminal domain
Splice variants
AR-V7 is an androgen receptor splice variant that can be detected in circulating tumor cells of metastatic prostate cancer patients. and is predictive of resistance to some drugs.
Ligands
| Compound | AR RBA (%) | |
|---|---|---|
| Metribolone | 100 | |
| Dihydrotestosterone | 85 | |
| Cyproterone acetate | 7.8 | |
| Bicalutamide | 1.4 | |
| Nilutamide | 0.9 | |
| Hydroxyflutamide | 0.57 | |
| Flutamide | <0 .0057="" span=""> | |
| Notes: Human prostate tissue used for the assays. Sources: See template. | ||
Agonists
- Endogenous androgens (e.g., testosterone, dihydrotestosterone, androstenedione, androstenediol, dehydroepiandrosterone)
- Synthetic androgens (e.g., methyltestosterone, metandienone, nandrolone, trenbolone, oxandrolone, stanozolol)
Mixed
Antagonists
- Steroidal antiandrogens (e.g., cyproterone acetate, chlormadinone acetate, spironolactone, oxendolone)
- Nonsteroidal antiandrogens (e.g., flutamide, nilutamide, bicalutamide, enzalutamide, apalutamide)
- N-Terminal domain antiandrogens (e.g., bisphenol A, EPI-001, ralaniten, JN compounds)
As a drug target
The AR is an important therapeutic target in prostate cancer. Thus many different antiandrogens have been developed, primarily targeting the ligand binding domain of the protein. AR ligands can either be classified based on their structure (steroidal or nonsteroidal) or based on their ability to activate or inhibit transcription (agonists or antagonists). Inhibitors that target alternative functional domains (N-terminal domain, DNA binding domain) of the protein are still under development.