| Wilson's disease | |
|---|---|
| Other names | Wilson disease, hepatolenticular degeneration |
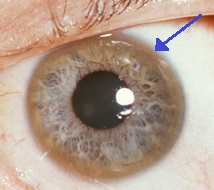 | |
| A brown ring on the edge of the cornea (Kayser–Fleischer ring) is common in Wilson's disease, especially when neurological symptoms are present. | |
| Specialty | Gastroenterology |
| Symptoms | Swelling of the legs, yellowish skin, personality changes |
| Usual onset | Age 5 to 35 |
| Causes | Genetic |
| Differential diagnosis | Chronic liver disease, Parkinson's disease, multiple sclerosis, others |
| Treatment | Dietary changes, chelating agents, zinc supplements, liver transplant |
| Frequency | ~1 per 30,000 |
Wilson's disease is a genetic disorder in which excess copper builds up in the body. Symptoms are typically related to the brain and liver. Liver-related symptoms include vomiting, weakness, fluid build up in the abdomen, swelling of the legs, yellowish skin and itchiness. Brain-related symptoms include tremors, muscle stiffness, trouble speaking, personality changes, anxiety and seeing or hearing things that others do not.
Wilson's disease is caused by a mutation in the Wilson disease protein (ATP7B) gene. This protein transports excess copper into bile, where it is excreted in waste products.[1] The condition is autosomal recessive; for a person to be affected, they must inherit a mutated copy of the gene from both parents. Diagnosis may be difficult and often involves a combination of blood tests, urine tests and a liver biopsy. Genetic testing may be used to screen family members of those affected.
Wilson's disease is typically treated with dietary changes and medication. Dietary changes involve eating a low-copper diet and not using copper cookware. Medications used include chelating agents such as trientine and d-penicillamine and zinc supplements.[1] Complications of Wilson's disease can include liver failure, liver cancer and kidney problems. A liver transplant may be helpful in those in whom other treatments are not effective or if liver failure occurs.
Wilson's disease occurs in about 1 in 30,000 people. Symptoms usually begin between the ages of 5 and 35 years. It was first described in 1854 by German pathologist Friedrich Theodor von Frerichs and is named after British neurologist Samuel Wilson.
Signs and symptoms
The main sites of copper accumulation are the liver and the brain, and consequently liver disease and neuropsychiatric symptoms are the main features that lead to diagnosis.
People with liver problems tend to come to medical attention earlier,
generally as children or teenagers, than those with neurological and
psychiatric symptoms, who tend to be in their twenties or older. Some
are identified only because relatives have been diagnosed with Wilson's
disease; many of these, when tested, turn out to have been experiencing
symptoms of the condition but have not received a diagnosis.
Liver disease
Liver disease may present itself as tiredness, increased bleeding tendency or confusion (due to hepatic encephalopathy) and portal hypertension. The latter, a condition in which the pressure in the portal vein is markedly increased, leads to esophageal varices, blood vessels in the esophagus that may bleed in a life-threatening fashion, as well as enlargement of the spleen (splenomegaly) and accumulation of fluid in the abdominal cavity (ascites). On examination, signs of chronic liver disease such as spider angiomata (small distended blood vessels, usually on the chest) may be observed. Chronic active hepatitis has caused cirrhosis of the liver in most by the time they develop symptoms. While most people with cirrhosis have an increased risk of hepatocellular carcinoma (liver cancer), this risk is relatively very low in Wilson's disease.
About 5% of all people are diagnosed only when they develop fulminant acute liver failure, often in the context of a hemolytic anemia (anemia due to the destruction of red blood cells). This leads to abnormalities in protein production (identified by deranged coagulation) and metabolism by the liver. The deranged protein metabolism leads to the accumulation of waste products such as ammonia in the bloodstream. When these irritate the brain, the person develops hepatic encephalopathy (confusion, coma, seizures and finally life-threatening swelling of the brain).
Neuropsychiatric symptoms
About
half the people with Wilson's disease have neurological or psychiatric
symptoms. Most initially have mild cognitive deterioration and
clumsiness, as well as changes in behavior. Specific neurological
symptoms usually then follow, often in the form of parkinsonism (cogwheel rigidity, bradykinesia or slowed movements and a lack of balance are the most common parkinsonian features) with or without a typical hand tremor, masked facial expressions, slurred speech, ataxia (lack of coordination) or dystonia (twisting and repetitive movements of part of the body). Seizures and migraine appear to be more common in Wilson's disease.
A characteristic tremor described as "wing-beating tremor" is
encountered in many people with Wilson's; this is absent at rest but can
be provoked by abducting the arms and flexing the elbows toward the
midline.
Cognition can also be affected in Wilson's disease. This comes in two, not mutually exclusive, categories: frontal lobe disorder (may present as impulsivity, impaired judgement, promiscuity, apathy and executive dysfunction with poor planning and decision making) and subcortical dementia (may present as slow thinking, memory loss and executive dysfunction, without signs of aphasia, apraxia or agnosia).
It is suggested that these cognitive involvements are related and
closely linked to psychiatric manifestations of the disease.
Psychiatric problems due to Wilson's disease may include behavioral changes, depression, anxiety disorders, and psychosis.
Psychiatric symptoms are commonly seen in conjunction with neurological
symptoms and are rarely manifested on their own. These symptoms are
often poorly defined and can sometimes be attributed to other causes.
Because of this, diagnosis of Wilson's disease is rarely made when only
psychiatric symptoms are present.
Other organ systems
Medical conditions have been linked with copper accumulation in Wilson's disease:
- Eyes: Kayser–Fleischer rings (KF rings), a pathognomonic sign, may be visible in the cornea of the eyes, either directly or on slit lamp examination as deposits of copper in a ring around the cornea. They are due to copper deposition in Descemet's membrane. They do not occur in all people with Wilson's disease. Wilson's disease is also associated with sunflower cataracts exhibited by brown or green pigmentation of the anterior and posterior lens capsule. Neither cause significant visual loss. KF rings occur in approximately 66% of diagnosed cases (more often in those with neurological symptoms rather than with liver problems).
- Kidneys: renal tubular acidosis (Type 2), a disorder of bicarbonate handling by the proximal tubules leads to nephrocalcinosis (calcium accumulation in the kidneys), a weakening of bones (due to calcium and phosphate loss), and occasionally aminoaciduria (loss of essential amino acids needed for protein synthesis).
- Heart: cardiomyopathy (weakness of the heart muscle) is a rare but recognized problem in Wilson's disease; it may lead to heart failure (fluid accumulation due to decreased pump function) and cardiac arrhythmias (episodes of irregular and/or abnormally fast or slow heart beat).
- Hormones: hypoparathyroidism (failure of the parathyroid glands leading to low calcium levels), infertility, and recurrent miscarriage.
Genetics
Wilson's disease has an autosomal recessive pattern of inheritance.
The Wilson's disease gene (ATP7B) is on chromosome 13 (13q14.3) and is expressed primarily in the liver, kidney, and placenta. The gene codes for a P-type (cation transport enzyme) ATPase that transports copper into bile and incorporates it into ceruloplasmin. Mutations can be detected in 90% of cases. Most (60%) are homozygous for ATP7B mutations (two abnormal copies), and 30% have only one abnormal copy. Ten percent have no detectable mutation.
Although 300 mutations of ATP7B have been described, in
most populations the cases of Wilson's disease are due to a small number
of mutations specific for that population. For instance, in Western
populations the H1069Q mutation (replacement of a histidine by a glutamine at position 1069 in the protein) is present in 37–63% of cases, while in China this mutation is very uncommon and R778L (arginine to leucine
at 778) is found more often. Relatively little is known about the
relative impact of various mutations, although the H1069Q mutation seems
to predict later onset and predominantly neurological problems,
according to some studies.
A normal variation in the PRNP
gene can modify the course of the disease by delaying the age of onset
and affecting the type of symptoms that develop. This gene produces prion protein, which is active in the brain and other tissues and also appears to be involved in transporting copper. A role for the ApoE gene was initially suspected but could not be confirmed.
The condition is inherited in an autosomal recessive pattern. In
order to inherit it, both of the parents of an individual must carry an
affected gene. Most have no family history of the condition.
People with only one abnormal gene are called carriers (heterozygotes)
and may have mild, but medically insignificant, abnormalities of copper
metabolism.
Wilson's disease is the most common from a group of hereditary diseases that cause copper overload in the liver. All can cause cirrhosis at a young age. The other members of the group are Indian childhood cirrhosis (ICC), endemic Tyrolean infantile cirrhosis and idiopathic copper toxicosis. These are not related to ATP7B mutations: for example, ICC has been linked to mutations in the KRT8 and the KRT18 gene.
Pathophysiology
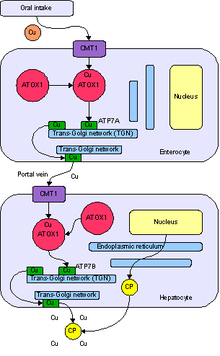
Normal absorption and distribution of copper. Cu = copper, CP = ceruloplasmin, green = ATP7B carrying copper.
Copper is needed by the body for a number of functions, predominantly as a cofactor for a number of enzymes such as ceruloplasmin, cytochrome c oxidase, dopamine β-hydroxylase, superoxide dismutase and tyrosinase.
Copper enters the body through the digestive tract. A transporter protein on the cells of the small bowel, copper membrane transporter 1 (Ctr1; SLC31A1), carries copper inside the cells, where some is bound to metallothionein and part is carried by ATOX1 to an organelle known as the trans-Golgi network. Here, in response to rising concentrations of copper, an enzyme called ATP7A (Menkes' protein) releases copper into the portal vein
to the liver. Liver cells also carry the CMT1 protein, and
metallothionein and ATOX1 bind it inside the cell, but here it is ATP7B
that links copper to ceruloplasmin and releases it into the bloodstream, as well as removing excess copper by secreting it into bile.
Both functions of ATP7B are impaired in Wilson's disease. Copper
accumulates in the liver tissue; ceruloplasmin is still secreted, but in
a form that lacks copper (termed apoceruloplasmin) and is rapidly
degraded in the bloodstream.
When the amount of copper in the liver overwhelms the proteins
that normally bind it, it causes oxidative damage through a process
known as Fenton chemistry; this damage eventually leads to chronic active hepatitis, fibrosis (deposition of connective tissue) and cirrhosis.
The liver also releases copper into the bloodstream that is not bound
to ceruloplasmin. This free copper precipitates throughout the body but
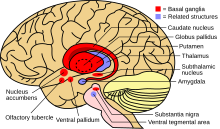
particularly in the kidneys, eyes and brain. In the brain, most copper
is deposited in the basal ganglia, particularly in the putamen and globus pallidus (together called the lenticular nucleus);
these areas normally participate in the coordination of movement as
well as playing a significant role in neurocognitive processes such as
the processing of stimuli and mood regulation. Damage to these areas,
again by Fenton chemistry, produces the neuropsychiatric symptoms seen
in Wilson's disease.
It is not clear why Wilson's disease causes hemolysis, but various lines of evidence suggest that a high level of free (non-ceruloplasmin bound) copper has a direct effect on either oxidation of hemoglobin, inhibition of energy-supplying enzymes in the red blood cell, or direct damage to the cell membrane.
Diagnosis
Location of the basal ganglia, the part of the brain affected by Wilson's disease
Wilson's disease may be suspected on the basis of any of the symptoms
mentioned above, or when a close relative has been found to have
Wilson's. Most have slightly abnormal liver function tests such as a raised aspartate transaminase, alanine transaminase and bilirubin level. If the liver damage is significant, albumin may be decreased due to an inability of damaged liver cells to produce this protein; likewise, the prothrombin time (a test of coagulation) may be prolonged as the liver is unable to produce proteins known as clotting factors. Alkaline phosphatase levels are relatively low in those with Wilson's-related acute liver failure. If there are neurological symptoms, magnetic resonance imaging (MRI) of the brain is usually performed; this shows hyperintensities in the part of the brain called the basal ganglia in the T2 setting. MRI may also demonstrate the characteristic "face of the giant panda" pattern.
There is no totally reliable test for Wilson's disease, but levels of ceruloplasmin
and copper in the blood, as well of the amount of copper excreted in
urine during a 24-hour period, are together used to form an impression
of the amount of copper in the body. The gold standard—or most ideal test—is a liver biopsy.
Ceruloplasmin
Ceruloplasmin
Levels of ceruloplasmin are abnormally low (<0 .2="" 80="" a="" at="" be="" can="" cases.="" g="" however="" href="https://en.wikipedia.org/wiki/Inflammation" in="" it="" levels="" nbsp="" normal="" of="" ongoing="" people="" present="" title="Inflammation" with="">inflammation
as it is an acute phase protein. Low ceruloplasmin is also found in Menkes disease and aceruloplasminemia, which are related to, but much rarer than Wilson's disease.
The combination of neurological symptoms, Kayser–Fleischer rings
and a low ceruloplasmin level is considered sufficient for the diagnosis
of Wilson's disease. In many cases, however, further tests are needed.
Serum and urine copper
Serum
copper is low, which may seem paradoxical given that Wilson's disease
is a disease of copper excess. However, 95% of plasma copper is carried
by ceruloplasmin which is often low in Wilson's disease. Urine copper is
elevated in Wilson's disease and is collected for 24 hours in a bottle
with a copper-free liner. Levels above 100 μg/24h (1.6 μmol/24h) confirm
Wilson's disease, and levels above 40 μg/24h (0.6 μmol/24h) are
strongly indicative. High urine copper levels are not unique to Wilson's disease; they are sometimes observed in autoimmune hepatitis and in cholestasis (any disease obstructing the flow of bile from the liver to the small bowel).
In children, the penicillamine
test may be used. A 500 mg oral dose of penicillamine is administered,
and urine collected for 24 hours. If this contains more than 1600 μg
(25 μmol), it is a reliable indicator of Wilson's disease. This test has not been validated in adults.
Liver biopsy
Once
other investigations have indicated Wilson's disease, the ideal test is
the removal of a small amount of liver tissue through a liver biopsy. This is assessed microscopically for the degree of steatosis and cirrhosis, and histochemistry and quantification of copper are used to measure the severity of the copper accumulation. A level of 250 μg
of copper per gram of dried liver tissue confirms Wilson's disease.
Occasionally, lower levels of copper are found; in that case, the
combination of the biopsy findings with all other tests could still lead
to a formal diagnosis of Wilson's.
In the earlier stages of the disease, the biopsy typically shows steatosis (deposition of fatty material), increased glycogen in the nucleus, and areas of necrosis
(cell death). In more advanced disease, the changes observed are quite
similar to those seen in autoimmune hepatitis, such as infiltration by inflammatory
cells, piecemeal necrosis and fibrosis (scar tissue). In advanced
disease, finally, cirrhosis is the main finding. In acute liver failure,
degeneration of the liver cells and collapse of the liver tissue
architecture is seen, typically on a background of cirrhotic changes.
Histochemical methods for detecting copper are inconsistent and
unreliable, and taken alone are regarded as insufficient to establish a
diagnosis.
Genetic testing
Mutation analysis of the ATP7B
gene, as well as other genes linked to copper accumulation in the
liver, may be performed. Once a mutation is confirmed, it is possible to
screen family members for the disease as part of clinical genetics family counseling.
Regional distributions of genes associated with Wilson's disease are
important to follow, as this can help clinicians design appropriate
screening strategies. Since mutations of the WD gene vary between
populations, research and genetic testing done in countries like the USA
or United Kingdom can pose problems as they tend to have more mixed
populations.
Treatment
Diet
In general, a diet low in copper-containing foods is recommended with the avoidance of mushrooms, nuts, chocolate, dried fruit, liver, sesame seeds and sesame oil, and shellfish.
Medication
Medical
treatments are available for Wilson's disease. Some increase the
removal of copper from the body, while others prevent the absorption of
copper from the diet.
Generally, penicillamine is the first treatment used. This binds copper (chelation)
and leads to excretion of copper in the urine. Hence, monitoring of the
amount of copper in the urine can be done to ensure a sufficiently high
dose is taken. Penicillamine is not without problems: about 20%
experience a side effect or complication of penicillamine treatment,
such as drug-induced lupus (causing joint pains and a skin rash) or myasthenia
(a nerve condition leading to muscle weakness). In those who presented
with neurological symptoms, almost half experience a paradoxical
worsening in their symptoms. While this phenomenon is observed in other
treatments for Wilson's, it is usually taken as an indication for
discontinuing penicillamine and commencing second-line treatment. Those intolerant to penicillamine may instead be commenced on trientine hydrochloride,
which also has chelating properties. Some recommend trientine as
first-line treatment, but experience with penicillamine is more
extensive. A further agent, under clinical investigation by Wilson Therapeutics, with known activity in Wilson's disease is tetrathiomolybdate. This is regarded as experimental, though some studies have shown a beneficial effect.
Once all results have returned to normal, zinc (usually in the form of a zinc acetate prescription called Galzin) may be used instead of chelators to maintain stable copper levels in the body. Zinc stimulates metallothionein,
a protein in gut cells that binds copper and prevents their absorption
and transport to the liver. Zinc therapy is continued unless symptoms
recur or if the urinary excretion of copper increases.
In rare cases where none of the oral treatments are effective, especially in severe neurological disease, dimercaprol (British anti-Lewisite) is occasionally necessary. This treatment is injected intramuscularly (into a muscle) every few weeks and has unpleasant side effects such as pain.
People who are asymptomatic
(for instance, those diagnosed through family screening or only as a
result of abnormal test results) are generally treated, as the copper
accumulation may cause long-term damage in the future. It is unclear
whether these people are best treated with penicillamine or zinc
acetate.
Physical and occupational therapies
Physiotherapy
and occupational therapy are beneficial for patients with the
neurologic form of the disease. The copper chelating treatment may take
up to six months to start working, and these therapies can assist in
coping with ataxia, dystonia, and tremors, as well as preventing the development of contractures that can result from dystonia.
Transplantation
Liver transplantation
is an effective cure for Wilson's disease but is used only in
particular scenarios because of the risks and complications associated
with the procedure. It is used mainly in people with fulminant
liver failure who fail to respond to medical treatment or in those with
advanced chronic liver disease. Liver transplantation is avoided in
severe neuropsychiatric illness, in which its benefit has not been
demonstrated.
Prognosis
Left
untreated, Wilson's disease tends to become progressively worse and is
eventually fatal. With early detection and treatment, most of those
affected can live relatively normal lives. Liver and neurologic damage
that occurs prior to treatment may improve, but it is often permanent.
History
The disease bears the name of the British physician Samuel Alexander Kinnier Wilson (1878–1937), a neurologist who described the condition, including the pathological changes in the brain and liver, in 1912. Wilson's work had been predated by, and drew on, reports from German neurologist Carl Westphal (in 1883), who termed it "pseudosclerosis"; by the British neurologist William Gowers (in 1888); by the Finnish neuropathologist Ernst Alexander Homén (in 1889-1892), who noted the hereditary nature of the disease; and by Adolph Strümpell (in 1898), who noted hepatic cirrhosis. Neuropathologist John Nathaniel Cumings made the link with copper accumulation in both the liver and the brain in 1948. The occurrence of hemolysis was noted in 1967.
Cumings, and simultaneously the New Zealand neurologist Derek Denny-Brown, working in the United States, first reported effective treatment with metal chelator British anti-Lewisite in 1951.
This treatment had to be injected but was one of the first therapies
available in the field of neurology, a field that classically was able
to observe and diagnose but had few treatments to offer. The first effective oral chelation agent, penicillamine, was discovered in 1956 by British neurologist John Walshe. In 1982, Walshe also introduced trientine, and was the first to develop tetrathiomolybdate for clinical use.
Zinc acetate therapy initially made its appearance in the Netherlands,
where physicians Schouwink and Hoogenraad used it in 1961 and in the
1970s, respectively, but it was further developed later by Brewer and
colleagues at the University of Michigan.
The genetic basis of Wilson's disease and linkage to ATP7B mutations was elucidated in the 1980s and 1990s by several research groups.
Other animals
Hereditary copper accumulation has been described in Bedlington Terriers, where it generally only affects the liver. It is due to mutations in the COMMD1 (or MURR1) gene. Despite this findings, COMMD1 mutations could not be detected in humans with non-Wilsonian copper accumulation states (such as Indian childhood cirrhosis) to explain their genetic origin.