The
central dogma of biology showing the flow of information from DNA to
the phenotype. Associated with each stage is the corresponding systems
biology tool, from genomics to metabolomics.
Metabolomics is the scientific study of chemical processes involving metabolites,
the small molecule substrates, intermediates and products of
metabolism. Specifically, metabolomics is the "systematic study of the
unique chemical fingerprints that specific cellular processes leave
behind", the study of their small-molecule metabolite profiles. The metabolome
represents the complete set of metabolites in a biological cell,
tissue, organ or organism, which are the end products of cellular
processes. mRNA gene expression data and proteomic
analyses reveal the set of gene products being produced in the cell,
data that represents one aspect of cellular function. Conversely,
metabolic profiling can give an instantaneous snapshot of the physiology
of that cell, and thus, metabolomics provides a direct "functional
readout of the physiological state" of an organism. One of the challenges of systems biology and functional genomics is to integrate genomics, transcriptomic, proteomic, and metabolomic information to provide a better understanding of cellular biology.
History
The
concept that individuals might have a "metabolic profile" that could be
reflected in the makeup of their biological fluids was introduced by
Roger Williams in the late 1940s, who used paper chromatography to suggest characteristic metabolic patterns in urine and saliva were associated with diseases such as schizophrenia.
However, it was only through technological advancements in the 1960s
and 1970s that it became feasible to quantitatively (as opposed to
qualitatively) measure metabolic profiles. The term "metabolic profile" was introduced by Horning, et al. in 1971 after they demonstrated that gas chromatography-mass spectrometry (GC-MS) could be used to measure compounds present in human urine and tissue extracts. The Horning group, along with that of Linus Pauling and Arthur B. Robinson led the development of GC-MS methods to monitor the metabolites present in urine through the 1970s.
Concurrently, NMR spectroscopy,
which was discovered in the 1940s, was also undergoing rapid advances.
In 1974, Seeley et al. demonstrated the utility of using NMR to detect
metabolites in unmodified biological samples. This first study on muscle highlighted the value of NMR in that it was determined that 90% of cellular ATP is complexed with magnesium. As sensitivity has improved with the evolution of higher magnetic field strengths and magic angle spinning, NMR continues to be a leading analytical tool to investigate metabolism. Recent efforts to utilize NMR for metabolomics have been largely driven by the laboratory of Jeremy K. Nicholson at Birkbeck College, University of London and later at Imperial College London. In 1984, Nicholson showed 1H
NMR spectroscopy could potentially be used to diagnose diabetes
mellitus, and later pioneered the application of pattern recognition
methods to NMR spectroscopic data.
In 1995 liquid chromatography mass spectrometry metabolomics experiments were performed by Gary Siuzdak while working with Richard Lerner
(then president of The Scripps Research Institute) and Benjamin
Cravatt, to analyze the cerebral spinal fluid from sleep deprived
animals. One molecule of particular interest, oleamide,
was observed and later shown to have sleep inducing properties. This
work is one of the earliest such experiments combining liquid
chromatography and mass spectrometry in metabolomics.
In 2005, the first metabolomics tandem mass spectrometry database, METLIN, for characterizing human metabolites was developed in the Siuzdak laboratory at The Scripps Research Institute.
METLIN has since grown and as of July 1, 2019, METLIN contains over
450,000 metabolites and other chemical entities, each compound having
experimental tandem mass spectrometry data generated from molecular
standards at multiple collision energies and in positive and negative
ionization modes. METLIN is the largest repository of tandem mass
spectrometry data of its kind.
In 2005, the Siuzdak lab was engaged in identifying metabolites
associated with sepsis and in an effort to address the issue of
statistically identifying the most relevant dysregulated metabolites
across hundreds of LC/MS datasets, the first algorithm was developed to
allow for the nonlinear alignment of mass spectrometry metabolomics
data. Called XCMS, where the "X" constitutes any chromatographic technology, it has since (2012) been developed as an online tool and as of 2019 (with METLIN) has over 30,000 registered users.
On 23 January 2007, the Human Metabolome Project, led by David Wishart of the University of Alberta,
Canada, completed the first draft of the human metabolome, consisting
of a database of approximately 2500 metabolites, 1200 drugs and 3500
food components. Similar projects have been underway in several plant species, most notably Medicago truncatula and Arabidopsis thaliana for several years.
As late as mid-2010, metabolomics was still considered an "emerging field".
Further, it was noted that further progress in the field depended in
large part, through addressing otherwise "irresolvable technical
challenges", by technical evolution of mass spectrometry instrumentation.
In 2015, real-time metabolome profiling was demonstrated for the first time.
Metabolome
Human metabolome project
Metabolome refers to the complete set of small-molecule (<1 .5="" a="" analogy="" and="" as="" be="" biological="" class="mw-redirect" coined="" found="" hormones="" href="https://en.wikipedia.org/wiki/Transcriptomics" in="" intermediates="" kda="" metabolic="" metabolites="" molecules="" organism.="" other="" sample="" secondary="" signaling="" single="" such="" the="" title="Transcriptomics" to="" was="" with="" within="" word="">transcriptomics
and proteomics;
like the transcriptome and the proteome, the metabolome is dynamic,
changing from second to second. Although the metabolome can be defined
readily enough, it is not currently possible to analyse the entire range
of metabolites by a single analytical method.
The first metabolite database (called METLIN) for searching fragmentation data from tandem mass spectrometry experiments was developed by the Siuzdak lab at The Scripps Research Institute in 2005.
METLIN contains over 450,000 metabolites and other chemical entities,
each compound having experimental tandem mass spectrometry data. In
2006,
the Siuzdak lab also developed the first algorithm to allow for the
nonlinear alignment of mass spectrometry metabolomics data. Called XCMS,
where the "X" constitutes any chromatographic technology, it has since
(2012) been developed as an online tool and as of 2019 (with METLIN) has over 30,000 registered users.
In January 2007, scientists at the University of Alberta and the University of Calgary
completed the first draft of the human metabolome. The Human Metabolome
Database (HMDB) is perhaps the most extensive public metabolomic
spectral database to date. The HMDB stores more than 40,000 different metabolite entries. They catalogued approximately 2500 metabolites, 1200 drugs and 3500 food components that can be found in the human body, as reported in the literature. This information, available at the Human Metabolome Database (www.hmdb.ca) and based on analysis of information available in the current scientific literature, is far from complete.
In contrast, much more is known about the metabolomes of other
organisms. For example, over 50,000 metabolites have been characterized
from the plant kingdom, and many thousands of metabolites have been
identified and/or characterized from single plants.
Each type of cell and tissue has a unique metabolic ‘fingerprint’
that can elucidate organ or tissue-specific information. Bio-specimens
used for metabolomics analysis include but not limit to plasma, serum,
urine, saliva, feces, muscle, sweat, exhaled breath and gastrointestinal
fluid.
The ease of collection facilitates high temporal resolution, and
because they are always at dynamic equilibrium with the body, they can
describe the host as a whole. Genome can tell what could happen, transcriptome can tell what appears to be happening, proteome can tell what makes it happen and metabolome can tell what has happened and what is happening.
Metabolites
Metabolites are the substrates, intermediates and products of metabolism. Within the context of metabolomics, a metabolite is usually defined as any molecule less than 1.5 kDa in size. However, there are exceptions to this depending on the sample and detection method. For example, macromolecules such as lipoproteins and albumin are reliably detected in NMR-based metabolomics studies of blood plasma.
In plant-based metabolomics, it is common to refer to "primary" and
"secondary" metabolites. A primary metabolite is directly involved in
the normal growth, development, and reproduction. A secondary metabolite is not directly involved in those processes, but usually has important ecological function. Examples include antibiotics and pigments. By contrast, in human-based metabolomics, it is more common to describe metabolites as being either endogenous (produced by the host organism) or exogenous. Metabolites of foreign substances such as drugs are termed xenometabolites.
The metabolome forms a large network of metabolic reactions, where outputs from one enzymatic chemical reaction are inputs to other chemical reactions. Such systems have been described as hypercycles.
Metabonomics
Metabonomics
is defined as "the quantitative measurement of the dynamic
multiparametric metabolic response of living systems to
pathophysiological stimuli or genetic modification". The word origin is
from the Greek μεταβολή meaning change and nomos meaning a rule set or set of laws. This approach was pioneered by Jeremy Nicholson at Murdoch University
and has been used in toxicology, disease diagnosis and a number of
other fields. Historically, the metabonomics approach was one of the
first methods to apply the scope of systems biology to studies of
metabolism.
There has been some disagreement over the exact differences
between 'metabolomics' and 'metabonomics'. The difference between the
two terms is not related to choice of analytical platform: although
metabonomics is more associated with NMR spectroscopy and metabolomics with mass spectrometry-based
techniques, this is simply because of usages amongst different groups
that have popularized the different terms. While there is still no
absolute agreement, there is a growing consensus that 'metabolomics'
places a greater emphasis on metabolic profiling at a cellular or organ
level and is primarily concerned with normal endogenous metabolism.
'Metabonomics' extends metabolic profiling to include information about
perturbations of metabolism caused by environmental factors (including
diet and toxins), disease processes, and the involvement of extragenomic
influences, such as gut microflora.
This is not a trivial difference; metabolomic studies should, by
definition, exclude metabolic contributions from extragenomic sources,
because these are external to the system being studied. However, in
practice, within the field of human disease research there is still a
large degree of overlap in the way both terms are used, and they are
often in effect synonymous.
Exometabolomics
Exometabolomics, or "metabolic footprinting", is the study of
extracellular metabolites. It uses many techniques from other subfields
of metabolomics, and has applications in biofuel development, bioprocessing, determining drugs' mechanism of action, and studying intercellular interactions.
Analytical technologies
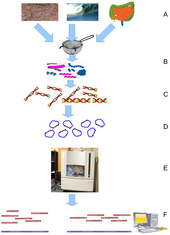
Key stages of a metabolomics study
The typical workflow of metabolomics studies is shown in the figure.
First, samples are collected from tissue, plasma, urine, saliva, cells,
etc. Next, metabolites extracted often with the addition of internal
standards and derivatization.
During sample analysis, metabolites are quantified (LC or GC coupled
with MS and/or NMR spectroscopy). The raw output data can be used for
metabolite feature extraction and further processed before statistical
analysis (such as PCA). Many bioinformatic tools and software are
available to identify associations with disease states and outcomes,
determine significant correlations, and characterize metabolic
signatures with existing biological knowledge.
Separation methods
Initially,
analytes in a metabolomic sample comprise a highly complex mixture.
This complex mixture can be simplified prior to detection by separating
some analytes from others. Separation achieves various goals: analytes
which cannot be resolved by the detector may be separated in this step;
in MS analysis ion suppression
is reduced; the retention time of the analyte serves as information
regarding its identity. This separation step is not mandatory and is
often omitted in NMR and "shotgun" based approaches such as shotgun lipidomics.
Gas chromatography (GC), especially when interfaced with mass spectrometry (GC-MS), is a widely used separation technique for metabolomic analysis.
GC offers very high chromatographic resolution, and can be used in
conjunction with a flame ionization detector (GC/FID) or a mass
spectrometer (GC-MS). The method is especially useful for identification
and quantification of small and volatile molecules.
However, a practical limitation of GC is the requirement of chemical
derivatization for many biomolecules as only volatile chemicals can be
analysed without derivatization. In cases where greater resolving power
is required, two-dimensional chromatography (GCxGC) can be applied.
High performance liquid chromatography (HPLC) has emerged as the most common separation technique for metabolomic analysis. With the advent of electrospray ionization, HPLC was coupled to MS. In contrast with GC,
HPLC has lower chromatographic resolution, but requires no
derivatization for polar molecules, and separates molecules in the
liquid phase. Additionally HPLC has the advantage that a much wider
range of analytes can be measured with a higher sensitivity than GC
methods.
Capillary electrophoresis
(CE) has a higher theoretical separation efficiency than HPLC (although
requiring much more time per separation), and is suitable for use with a
wider range of metabolite classes than is GC. As for all
electrophoretic techniques, it is most appropriate for charged analytes.
Detection methods
Comparison of most common used metabolomics methods
Mass spectrometry (MS) is used to identify and to quantify metabolites after optional separation by GC, HPLC (LC-MS), or CE. GC-MS
was the first hyphenated technique to be developed. Identification
leverages the distinct patterns in which analytes fragment which can be
thought of as a mass spectral fingerprint; libraries exist that allow
identification of a metabolite according to this fragmentation pattern.
MS is both sensitive and can be very specific. There are also a number
of techniques which use MS as a stand-alone technology: the sample is
infused directly into the mass spectrometer with no prior separation,
and the MS provides sufficient selectivity to both separate and to
detect metabolites.
For analysis by mass spectrometry the analytes must be imparted with a charge and transferred to the gas phase. Electron ionization
(EI) is the most common ionization technique applies to GC separations
as it is amenable to low pressures. EI also produces fragmentation of
the analyte, both providing structural information while increasing the
complexity of the data and possibly obscuring the molecular ion. Atmospheric-pressure chemical ionization
(APCI) is an atmospheric pressure technique that can be applied to all
the above separation techniques. APCI is a gas phase ionization method
slightly more aggressive ionization than ESI which is suitable for less
polar compounds. Electrospray ionization
(ESI) is the most common ionization technique applied in LC/MS. This
soft ionization is most successful for polar molecules with ionizable
functional groups. Another commonly used soft ionization technique is secondary electrospray ionization (SESI).
Surface-based mass analysis has seen a resurgence in the past
decade, with new MS technologies focused on increasing sensitivity,
minimizing background, and reducing sample preparation. The ability to
analyze metabolites directly from biofluids and tissues continues to
challenge current MS technology, largely because of the limits imposed
by the complexity of these samples, which contain thousands to tens of
thousands of metabolites. Among the technologies being developed to
address this challenge is Nanostructure-Initiator MS (NIMS),
a desorption/ ionization approach that does not require the application
of matrix and thereby facilitates small-molecule (i.e., metabolite)
identification. MALDI
is also used however, the application of a MALDI matrix can add
significant background at <1000 achieved="" addition="" analysis="" and="" applied="" approaches="" be="" because="" been="" biofluids="" can="" complicates="" crystals="" da="" desorption="" have="" i.e.="" imaging.="" in="" ionization="" limitations="" limits="" low-mass="" matrix-free="" matrix="" metabolites="" of="" other="" p="" range="" resolution="" resulting="" several="" size="" spatial="" that="" the="" these="" tissue="" tissues.="" to="">
Secondary ion mass spectrometry (SIMS) was one of the first matrix-free desorption/ionization approaches used to analyze metabolites from biological samples.
SIMS uses a high-energy primary ion beam to desorb and generate
secondary ions from a surface. The primary advantage of SIMS is its high
spatial resolution (as small as 50 nm), a powerful characteristic for
tissue imaging with MS. However, SIMS has yet to be readily applied to
the analysis of biofluids and tissues because of its limited sensitivity
at >500 Da and analyte fragmentation generated by the high-energy
primary ion beam. Desorption electrospray ionization
(DESI) is a matrix-free technique for analyzing biological samples that
uses a charged solvent spray to desorb ions from a surface. Advantages
of DESI are that no special surface is required and the analysis is
performed at ambient pressure with full access to the sample during
acquisition. A limitation of DESI is spatial resolution because
"focusing" the charged solvent spray is difficult. However, a recent
development termed laser ablation ESI (LAESI) is a promising approach to circumvent this limitation. Most recently, ion trap techniques such as orbitrap mass spectrometry are also applied to metabolomics research.
Nuclear magnetic resonance
(NMR) spectroscopy is the only detection technique which does not rely
on separation of the analytes, and the sample can thus be recovered for
further analyses. All kinds of small molecule metabolites can be
measured simultaneously - in this sense, NMR is close to being a
universal detector. The main advantages of NMR are high analytical
reproducibility and simplicity of sample preparation. Practically,
however, it is relatively insensitive compared to mass
spectrometry-based techniques. Comparison of most common used metabolomics methods is shown in the table.
Although NMR and MS are the most widely used, modern day
techniques other methods of detection that have been used. These include
Fourier-transform ion cyclotron resonance, ion-mobility spectrometry, electrochemical detection (coupled to HPLC), Raman spectroscopy and radiolabel (when combined with thin-layer chromatography).
Statistical methods
Software List for Metabolomic Analysis
The data generated in metabolomics usually consist of measurements
performed on subjects under various conditions. These measurements may
be digitized spectra, or a list of metabolite features. In its simplest
form this generates a matrix with rows corresponding to subjects and
columns corresponding with metabolite features (or vice versa). Several statistical programs are currently available for analysis of both NMR and mass spectrometry
data. A great number of free software are already available for the
analysis of metabolomics data shown in the table. Some statistical tools
listed in the table were designed for NMR data analyses were also
useful for MS data.
For mass spectrometry data, software is available that identifies
molecules that vary in subject groups on the basis of mass-over-charge
value and sometimes retention time depending on the experimental design.
Once metabolite data matrix is determined, unsupervised data
reduction techniques (e.g. PCA) can be used to elucidate patterns and
connections. In many studies, including those evaluating drug-toxicity
and some disease models, the metabolites of interest are not known a priori.
This makes unsupervised methods, those with no prior assumptions of
class membership, a popular first choice. The most common of these
methods includes principal component analysis (PCA) which can efficiently reduce the dimensions of a dataset to a few which explain the greatest variation.
When analyzed in the lower-dimensional PCA space, clustering of samples
with similar metabolic fingerprints can be detected. PCA algorithms aim
to replace all correlated variables by a much smaller number of
uncorrelated variables (referred to as principal components (PCs)) and
retain most of the information in the original dataset.[59]
This clustering can elucidate patterns and assist in the determination
of disease biomarkers - metabolites that correlate most with class
membership.
Linear models are commonly used for metabolomics data, but are affected by multicollinearity. On the other hand, multivariate statistics are thriving methods for high-dimensional correlated metabolomics data, of which the most popular one is Projection to Latent Structures (PLS) regression and its classification version PLS-DA. Other data mining methods, such as random forest, support-vector machine, k-nearest neighbors
etc. are received increasing attention for untargeted metabolomics data
analysis. In the case of univariate methods, variables are analyzed one
by one using classical statistics tools (such as Student's t-test, ANOVA or mixed models) and only these with sufficient small p-values are considered relevant. However, correction strategies should be used to reduce false discoveries when multiple comparisons are conducted. For multivariate analysis, models should always be validated to ensure the results can be generalized.
Key applications
Toxicity assessment/toxicology
by metabolic profiling (especially of urine or blood plasma samples)
detects the physiological changes caused by toxic insult of a chemical
(or mixture of chemicals). In many cases, the observed changes can be
related to specific syndromes, e.g. a specific lesion in liver or
kidney. This is of particular relevance to pharmaceutical companies
wanting to test the toxicity of potential drug candidates: if a compound can be eliminated before it reaches clinical trials on the grounds of adverse toxicity, it saves the enormous expense of the trials.
For functional genomics, metabolomics can be an excellent tool for determining the phenotype
caused by a genetic manipulation, such as gene deletion or insertion.
Sometimes this can be a sufficient goal in itself—for instance, to
detect any phenotypic changes in a genetically modified plant intended
for human or animal consumption. More exciting is the prospect of
predicting the function of unknown genes
by comparison with the metabolic perturbations caused by
deletion/insertion of known genes. Such advances are most likely to come
from model organisms such as Saccharomyces cerevisiae and Arabidopsis thaliana. The Cravatt laboratory at The Scripps Research Institute has recently applied this technology to mammalian systems, identifying the N-acyltaurines as previously uncharacterized endogenous substrates for the enzyme fatty acid amide hydrolase (FAAH) and the monoalkylglycerol ethers (MAGEs) as endogenous substrates for the uncharacterized hydrolase KIAA1363.
Metabologenomics is a novel approach to integrate metabolomics
and genomics data by correlating microbial-exported metabolites with
predicted biosynthetic genes.
This bioinformatics-based pairing method enables natural product
discovery at a larger-scale by refining non-targeted metabolomic
analyses to identify small molecules with related biosynthesis and to
focus on those that may not have previously well known structures.
Fluxomics
is a further development of metabolomics. The disadvantage of
metabolomics is that it only provides the user with steady-state level
information, while fluxomics determines the reaction rates of metabolic
reactions and can trace metabolites in a biological system over time.
Nutrigenomics
is a generalised term which links genomics, transcriptomics, proteomics
and metabolomics to human nutrition. In general a metabolome in a given
body fluid is influenced by endogenous factors such as age, sex, body
composition and genetics as well as underlying pathologies. The large
bowel microflora are also a very significant potential confounder of
metabolic profiles and could be classified as either an endogenous or
exogenous factor. The main exogenous factors are diet and drugs. Diet
can then be broken down to nutrients and non- nutrients. Metabolomics
is one means to determine a biological endpoint, or metabolic
fingerprint, which reflects the balance of all these forces on an
individual's metabolism.