From Wikipedia, the free encyclopedia
When
liquid oxygen is poured from a beaker into a strong magnet, the oxygen
is temporarily contained between the magnetic poles owing to its
paramagnetism.
Paramagnetism is a form of
magnetism whereby some materials are weakly attracted by an externally applied
magnetic field, and form internal,
induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior,
diamagnetic
materials are repelled by magnetic fields and form induced magnetic
fields in the direction opposite to that of the applied magnetic field. Paramagnetic materials include most
chemical elements and some
compounds; they have a relative
magnetic permeability slightly greater than 1 (i.e., a small positive
magnetic susceptibility) and hence are attracted to magnetic fields. The
magnetic moment
induced by the applied field is linear in the field strength and rather
weak. It typically requires a sensitive analytical balance to detect
the effect and modern measurements on paramagnetic materials are often
conducted with a
SQUID magnetometer.
Paramagnetism is due to the presence of unpaired electrons in the material, so most atoms with incompletely filled atomic orbitals are paramagnetic, although exceptions such as copper exist. Due to their spin, unpaired electrons have a magnetic dipole moment
and act like tiny magnets. An external magnetic field causes the
electrons' spins to align parallel to the field, causing a net
attraction. Paramagnetic materials include aluminium, oxygen, titanium, and iron oxide (FeO). Therefore, a simple rule of thumb is used in chemistry to determine whether a particle (atom, ion, or molecule) is paramagnetic or diamagnetic:
if all electrons in the particle are paired, then the substance made of
this particle is diamagnetic; if it has unpaired electrons, then the
substance is paramagnetic.
Unlike ferromagnets, paramagnets do not retain any magnetization in the absence of an externally applied magnetic field because thermal motion randomizes the spin orientations. (Some paramagnetic materials retain spin disorder even at absolute zero, meaning they are paramagnetic in the ground state,
i.e. in the absence of thermal motion.) Thus the total magnetization
drops to zero when the applied field is removed. Even in the presence of
the field there is only a small induced magnetization because only a
small fraction of the spins will be oriented by the field. This fraction
is proportional to the field strength and this explains the linear
dependency. The attraction experienced by ferromagnetic materials is
non-linear and much stronger, so that it is easily observed, for
instance, in the attraction between a refrigerator magnet and the iron of the refrigerator itself.
Relation to electron spins
Constituent atoms or molecules of paramagnetic materials have permanent magnetic moments (dipoles), even in the absence of an applied field. The permanent moment generally is due to the spin of unpaired electrons in atomic or molecular electron orbitals. In pure paramagnetism, the dipoles
do not interact with one another and are randomly oriented in the
absence of an external field due to thermal agitation, resulting in zero
net magnetic moment. When a magnetic field is applied, the dipoles will
tend to align with the applied field, resulting in a net magnetic
moment in the direction of the applied field. In the classical
description, this alignment can be understood to occur due to a torque
being provided on the magnetic moments by an applied field, which tries
to align the dipoles parallel to the applied field. However, the true
origins of the alignment can only be understood via the quantum-mechanical properties of spin and angular momentum.
If there is sufficient energy exchange between neighbouring
dipoles, they will interact, and may spontaneously align or anti-align
and form magnetic domains, resulting in ferromagnetism (permanent magnets) or antiferromagnetism, respectively. Paramagnetic behavior can also be observed in ferromagnetic materials that are above their Curie temperature, and in antiferromagnets above their Néel temperature. At these temperatures, the available thermal energy simply overcomes the interaction energy between the spins.
In general, paramagnetic effects are quite small: the magnetic susceptibility is of the order of 10−3 to 10−5 for most paramagnets, but may be as high as 10−1 for synthetic paramagnets such as ferrofluids.
Delocalization
In conductive materials, the electrons are delocalized, that is, they travel through the solid more or less as free electrons. Conductivity can be understood in a band structure
picture as arising from the incomplete filling of energy bands.
In an ordinary nonmagnetic conductor the conduction band is identical
for both spin-up and spin-down electrons. When a magnetic field is
applied, the conduction band splits apart into a spin-up and a spin-down
band due to the difference in magnetic potential energy for spin-up and spin-down electrons.
Since the Fermi level
must be identical for both bands, this means that there will be a small
surplus of the type of spin in the band that moved downwards. This
effect is a weak form of paramagnetism known as Pauli paramagnetism.
The effect always competes with a diamagnetic
response of opposite sign due to all the core electrons of the atoms.
Stronger forms of magnetism usually require localized rather than
itinerant electrons. However, in some cases a band structure can result
in which there are two delocalized sub-bands with states of opposite
spins that have different energies. If one subband is preferentially
filled over the other, one can have itinerant ferromagnetic order. This
situation usually only occurs in relatively narrow (d-)bands, which are
poorly delocalized.
s and p electrons
Generally,
strong delocalization in a solid due to large overlap with neighboring
wave functions means that there will be a large Fermi velocity;
this means that the number of electrons in a band is less sensitive to
shifts in that band's energy, implying a weak magnetism. This is why s-
and p-type metals are typically either Pauli-paramagnetic or as in the
case of gold even diamagnetic. In the latter case the diamagnetic
contribution from the closed shell inner electrons simply wins over the
weak paramagnetic term of the almost free electrons.
d and f electrons
Stronger
magnetic effects are typically only observed when d or f electrons are
involved. Particularly the latter are usually strongly localized.
Moreover, the size of the magnetic moment on a lanthanide atom can be
quite large as it can carry up to 7 unpaired electrons in the case of gadolinium(III) (hence its use in MRI). The high magnetic moments associated with lanthanides is one reason why superstrong magnets are typically based on elements like neodymium or samarium.
Molecular localization
The above picture is a generalization
as it pertains to materials with an extended lattice rather than a
molecular structure. Molecular structure can also lead to localization
of electrons. Although there are usually energetic reasons why a
molecular structure results such that it does not exhibit partly filled
orbitals (i.e. unpaired spins), some non-closed shell moieties do occur
in nature. Molecular oxygen is a good example. Even in the frozen solid
it contains di-radical molecules
resulting in paramagnetic behavior. The unpaired spins reside in
orbitals derived from oxygen p wave functions, but the overlap is
limited to the one neighbor in the O2 molecules. The
distances to other oxygen atoms in the lattice remain too large to lead
to delocalization and the magnetic moments remain unpaired.
Theory
The Bohr–Van Leeuwen theorem
proves that there cannot be any diamagnetism or paramagnetism in a
purely classical system. The paramagnetic response has then two possible
quantum origins, either coming from permanent magnetic moments of the
ions or from the spatial motion of the conduction electrons inside the
material. Both description are given below.
Curie's law
For low levels of magnetization, the magnetization of paramagnets follows what is known as Curie's law, at least approximately. This law indicates that the susceptibility,  ,
of paramagnetic materials is inversely proportional to their
temperature, i.e. that materials become more magnetic at lower
temperatures. The mathematical expression is:
,
of paramagnetic materials is inversely proportional to their
temperature, i.e. that materials become more magnetic at lower
temperatures. The mathematical expression is:

where:
 is the resulting magnetization, measured in amperes/meter (A/m),
is the resulting magnetization, measured in amperes/meter (A/m), is the volume magnetic susceptibility (dimensionless),
is the volume magnetic susceptibility (dimensionless), is the auxiliary magnetic field (A/m),
is the auxiliary magnetic field (A/m), is absolute temperature, measured in kelvins (K),
is absolute temperature, measured in kelvins (K), is a material-specific Curie constant (K).
is a material-specific Curie constant (K).
Curie's law is valid under the commonly encountered conditions of low magnetization (μBH ≲ kBT), but does not apply in the high-field/low-temperature regime where saturation of magnetization occurs (μBH ≳ kBT)
and magnetic dipoles are all aligned with the applied field. When the
dipoles are aligned, increasing the external field will not increase the
total magnetization since there can be no further alignment.
For a paramagnetic ion with noninteracting magnetic moments with angular momentum J, the Curie constant is related the individual ions' magnetic moments,

where n is the number of atoms per unit volume. The parameter μeff
is interpreted as the effective magnetic moment per paramagnetic ion.
If one uses a classical treatment with molecular magnetic moments
represented as discrete magnetic dipoles, μ, a Curie Law expression of the same form will emerge with μ appearing in place of μeff.
When orbital angular momentum contributions to the magnetic moment are small, as occurs for most organic radicals or for octahedral transition metal complexes with d3 or high-spin d5 configurations, the effective magnetic moment takes the form ( with g-factor ge = 2.0023... ≈ 2),

where Nu is the number of unpaired electrons. In other transition metal complexes this yields a useful, if somewhat cruder, estimate.
When Curie constant is null, second order effects that couple the
ground state with the excited states can also lead to a paramagnetic
susceptibility independent of the temperature, known as Van Vleck susceptibility.
Pauli paramagnetism
For some alkali metals and noble metals, conduction electrons are weakly interacting and delocalized in space forming a Fermi gas.
For these materials one contribution to the magnetic response comes
from the interaction between the electron spins and the magnetic field
known as Pauli paramagnetism. For a small magnetic field  , the additional energy per electron from the interaction between an electron spin and the magnetic field is given by:
, the additional energy per electron from the interaction between an electron spin and the magnetic field is given by:

where  is the vacuum permeability,
is the vacuum permeability,  is the electron magnetic moment,
is the electron magnetic moment,  is the Bohr magneton,
is the Bohr magneton,  is the reduced Planck constant, and the g-factor cancels with the spin
is the reduced Planck constant, and the g-factor cancels with the spin  . The
. The  indicates that the sign is positive (negative) when the electron spin component in the direction of
indicates that the sign is positive (negative) when the electron spin component in the direction of  is parallel (antiparallel) to the magnetic field.
is parallel (antiparallel) to the magnetic field.
In
a metal, the application of an external magnetic field increases the
density of electrons with spins antiparallel with the field and lowers
the density of the electrons with opposite spin. Note: The arrows in
this picture indicate spin direction, not magnetic moment.
For low temperatures with respect to the Fermi temperature  (around 104 kelvins for metals), the number density of electrons
(around 104 kelvins for metals), the number density of electrons  (
( ) pointing parallel (antiparallel) to the magnetic field can be written as:
) pointing parallel (antiparallel) to the magnetic field can be written as:

with  the total free-electrons density and
the total free-electrons density and  the electronic density of states (number of states per energy per volume) at the Fermi energy
the electronic density of states (number of states per energy per volume) at the Fermi energy  .
.
In this approximation the magnetization is given as the magnetic moment of one electron times the difference in densities:

which yields a positive paramagnetic susceptibility independent of temperature:

The Pauli paramagnetic susceptibility is a macroscopic effect and has to be contrasted with Landau diamagnetic susceptibility
which is equal to minus one third of Pauli's and also comes from
delocalized electrons. The Pauli susceptibility comes from the spin
interaction with the magnetic field while the Landau susceptibility
comes from the spatial motion of the electrons and it is independent of
the spin. In doped semiconductors the ratio between Landau's and Pauli's
susceptibilities changes as the effective mass of the charge carriers  can differ from the electron mass
can differ from the electron mass  .
.
The magnetic response calculated for a gas of electrons is not
the full picture as the magnetic susceptibility coming from the ions has
to be included. Additionally, this formulas may break down for confined
systems that differ from the bulk, like quantum dots, or for high fields, as demonstrated in the De Haas-Van Alphen effect.
Pauli paramagnetism is named after the physicist Wolfgang Pauli. Before Pauli's theory, the lack of a strong Curie paramagnetism in metals was an open problem as the leading model could not account for this contribution without the use of quantum statistics.
Examples of paramagnets
Materials
that are called "paramagnets" are most often those that exhibit, at
least over an appreciable temperature range, magnetic susceptibilities
that adhere to the Curie or Curie–Weiss laws. In principle any system
that contains atoms, ions, or molecules with unpaired spins can be
called a paramagnet, but the interactions between them need to be
carefully considered.
Systems with minimal interactions
The narrowest definition would be: a system with unpaired spins that do not interact with each other. In this narrowest sense, the only pure paramagnet is a dilute gas of monatomic hydrogen atoms. Each atom has one non-interacting unpaired electron.
A gas of lithium atoms already possess two paired core electrons
that produce a diamagnetic response of opposite sign. Strictly speaking
Li is a mixed system therefore, although admittedly the diamagnetic
component is weak and often neglected. In the case of heavier elements
the diamagnetic contribution becomes more important and in the case of
metallic gold it dominates the properties. The element hydrogen is
virtually never called 'paramagnetic' because the monatomic gas is
stable only at extremely high temperature; H atoms combine to form
molecular H2 and in so doing, the magnetic moments are lost (quenched), because of the spins pair. Hydrogen is therefore diamagnetic
and the same holds true for many other elements. Although the
electronic configuration of the individual atoms (and ions) of most
elements contain unpaired spins, they are not necessarily paramagnetic,
because at ambient temperature quenching is very much the rule rather
than the exception. The quenching tendency is weakest for f-electrons
because f (especially 4f) orbitals are radially contracted
and they overlap only weakly with orbitals on adjacent atoms.
Consequently, the lanthanide elements with incompletely filled
4f-orbitals are paramagnetic or magnetically ordered.
μeff values for typical d3 and d5 transition metal complexes.
| [Cr(NH3)6]Br3 |
3.77
|
| K3[Cr(CN)6] |
3.87
|
| K3[MoCl6] |
3.79
|
| K4[V(CN)6] |
3.78
|
| [Mn(NH3)6]Cl2 |
5.92
|
| (NH4)2[Mn(SO4)2]·6H2O |
5.92
|
| NH4[Fe(SO4)2]·12H2O |
5.89
|
Thus, condensed phase paramagnets are only possible if the
interactions of the spins that lead either to quenching or to ordering
are kept at bay by structural isolation of the magnetic centers. There
are two classes of materials for which this holds:
- Molecular materials with a (isolated) paramagnetic center.
- Good examples are coordination complexes of d- or f-metals or proteins with such centers, e.g. myoglobin. In such materials the organic part of the molecule acts as an envelope shielding the spins from their neighbors.
- Small molecules can be stable in radical form, oxygen O2 is a good example. Such systems are quite rare because they tend to be rather reactive.
- Dilute systems.
- Dissolving a paramagnetic species in a diamagnetic lattice at small concentrations, e.g. Nd3+ in CaCl2
will separate the neodymium ions at large enough distances that they do
not interact. Such systems are of prime importance for what can be
considered the most sensitive method to study paramagnetic systems: EPR.
Systems with interactions
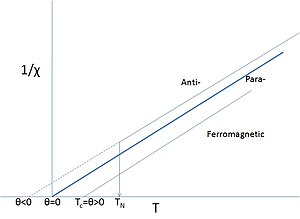
Idealized Curie–Weiss behavior; N.B. TC=θ, but TN is not θ. Paramagnetic regimes are denoted by solid lines. Close to TN or TC the behavior usually deviates from ideal.
As stated above, many materials that contain d- or f-elements do
retain unquenched spins. Salts of such elements often show paramagnetic
behavior but at low enough temperatures the magnetic moments may order.
It is not uncommon to call such materials 'paramagnets', when referring
to their paramagnetic behavior above their Curie or Néel-points,
particularly if such temperatures are very low or have never been
properly measured. Even for iron it is not uncommon to say that iron becomes a paramagnet above its relatively high Curie-point. In that case the Curie-point is seen as a phase transition
between a ferromagnet and a 'paramagnet'. The word paramagnet now
merely refers to the linear response of the system to an applied field,
the temperature dependence of which requires an amended version of
Curie's law, known as the Curie–Weiss law:

This amended law includes a term θ that describes the exchange
interaction that is present albeit overcome by thermal motion. The sign
of θ depends on whether ferro- or antiferromagnetic interactions
dominate and it is seldom exactly zero, except in the dilute, isolated
cases mentioned above.
Obviously, the paramagnetic Curie–Weiss description above TN or TC is a rather different interpretation of the word "paramagnet" as it does not imply the absence of interactions, but rather that the magnetic structure is random in the absence of an external field at these sufficiently high temperatures. Even if θ
is close to zero this does not mean that there are no interactions,
just that the aligning ferro- and the anti-aligning antiferromagnetic
ones cancel. An additional complication is that the interactions are
often different in different directions of the crystalline lattice (anisotropy), leading to complicated magnetic structures once ordered.
Randomness of the structure also applies to the many metals that
show a net paramagnetic response over a broad temperature range. They do
not follow a Curie type law as function of temperature however, often
they are more or less temperature independent. This type of behavior is
of an itinerant nature and better called Pauli-paramagnetism, but it is
not unusual to see, for example, the metal aluminium called a "paramagnet", even though interactions are strong enough to give this element very good electrical conductivity.
Superparamagnets
Some
materials show induced magnetic behavior that follows a Curie type law
but with exceptionally large values for the Curie constants. These
materials are known as
superparamagnets.
They are characterized by a strong ferromagnetic or ferrimagnetic type
of coupling into domains of a limited size that behave independently
from one another. The bulk properties of such a system resembles that of
a paramagnet, but on a microscopic level they are ordered. The
materials do show an ordering temperature above which the behavior
reverts to ordinary paramagnetism (with interaction).
Ferrofluids
are a good example, but the phenomenon can also occur inside solids,
e.g., when dilute paramagnetic centers are introduced in a strong
itinerant medium of ferromagnetic coupling such as when Fe is
substituted in TlCu
2Se
2 or the alloy AuFe. Such
systems contain ferromagnetically coupled clusters that freeze out at
lower temperatures. They are also called
mictomagnets.