A
fungus (
;
plural: fungi
[3] or funguses
[4]) is any member of the group of
eukaryotic organisms that includes unicellular microorganisms such as
yeasts and
molds, as well as multicellular fungi that produce familiar fruiting forms known as
mushrooms. These organisms are classified as a
kingdom,
Fungi, which is separate from the other life kingdoms of
plants,
animals,
protists, and
bacteria. One difference that places fungi in a different kingdom is that its
cell walls contain
chitin, unlike the cell walls of plants, bacteria and some protists. Similar to animals, fungi are
heterotrophs, that is, they acquire their food by absorbing dissolved molecules, typically by secreting
digestive enzymes into their environment. Growth is their means of mobility, except for spores, which may travel through the air or water (a few of which are flagellated). Fungi are the principal decomposers in ecological systems. These and other differences place fungi in a single group of related organisms, named the
Eumycota (
true fungi or
Eumycetes), that share a
common ancestor (is a
monophyletic group). This fungal group is distinct from the structurally similar
myxomycetes (slime molds) and
oomycetes (water molds). The discipline of
biology devoted to the study of fungi is known as
mycology (from the
Greek μύκης, mukēs, meaning "fungus"). In the past, mycology was regarded as a branch of
botany; today it is a separate kingdom in biological
taxonomy. Genetic studies have shown that fungi are more closely related to animals than to plants.
Abundant worldwide, most fungi are inconspicuous because of the small size of their structures, and their
cryptic lifestyles in soil, on dead matter. They are both
symbionts of plants, animals, or other fungi and also
parasites. They may become noticeable when
fruiting, either as mushrooms or as molds. Fungi perform an essential role in the decomposition of organic matter and have fundamental roles in
nutrient cycling and exchange in the environment. They have long been used as a direct source of food, in the form of mushrooms and
truffles, as a
leavening agent for bread, in the
fermentation of various food products, such as
wine,
beer, and
soy sauce. Since the 1940s, fungi have been used for the production of
antibiotics, and, more recently, various
enzymes produced by fungi are used
industrially and in
detergents. Fungi are also used as
biological pesticides to control weeds, plant diseases and insect pests. Many species produce
bioactive compounds called
mycotoxins, such as
alkaloids and
polyketides, that are toxic to animals including humans. The fruiting structures of a few species contain
psychotropic compounds and are consumed
recreationally or in traditional
spiritual ceremonies. Fungi can break down manufactured materials and buildings, and become significant
pathogens of humans and other animals. Losses of crops due to fungal diseases (e.g.,
rice blast disease) or food
spoilage can have a large impact on human
food supplies and local economies.
The fungus kingdom encompasses an enormous diversity of
taxa with varied ecologies,
life cycle strategies, and
morphologies ranging from unicellular aquatic
chytrids to large mushrooms. However, little is known of the true
biodiversity of Kingdom Fungi, which has been estimated at 1.5 million to 5 million species, with about 5% of these having been formally classified. Ever since the pioneering 18th and 19th century
taxonomical works of
Carl Linnaeus,
Christian Hendrik Persoon, and
Elias Magnus Fries, fungi have been
classified according to their morphology (e.g., characteristics such as spore color or microscopic features) or
physiology. Advances in
molecular genetics have opened the way for
DNA analysis to be incorporated into taxonomy, which has sometimes challenged the historical groupings based on morphology and other traits.
Phylogenetic studies published in the last decade have helped reshape the classification of Kingdom Fungi, which is divided into one
subkingdom, seven
phyla, and ten subphyla. The classification of fungi is based largely on the characteristics of their spores and spore-bearing structures.
Etymology
The English word
fungus is directly adopted from the
Latin fungus (mushroom), used in the writings of
Horace and
Pliny.
[5] This in turn is derived from the
Greek word
sphongos (σφογγος "sponge"), which refers to the
macroscopic structures and morphology of mushrooms and molds;
[6] the root is also used in other languages, such as the German
Schwamm ("sponge") and
Schimmel ("mold").
[7] The use of the word
mycology, which is derived from the Greek
mykes (μύκης "mushroom") and
logos (λόγος "discourse"),
[8] to denote the scientific study of fungi is thought to have originated in 1836 with English naturalist
Miles Joseph Berkeley's publication
The English Flora of Sir James Edward Smith, Vol. 5.[6] A group of all the fungi present in a particular area or geographic region is known as
mycobiota (plural noun, no singular), e.g., "the mycobiota of Ireland".
[9]
Characteristics
Before the introduction of
molecular methods for phylogenetic analysis,
taxonomists considered fungi to be members of the
plant kingdom because of similarities in lifestyle: both fungi and plants are mainly
immobile, and have similarities in general morphology and growth habitat. Like plants, fungi often grow in soil, and in the case of
mushrooms form conspicuous
fruit bodies, which sometimes resemble plants such as
mosses. The fungi are now considered a separate kingdom, distinct from both plants and animals, from which they appear to have
diverged around one billion years ago.
[10][11] Some morphological, biochemical, and genetic features are shared with other organisms, while others are unique to the fungi, clearly separating them from the other kingdoms:
Shared features:
- With other eukaryotes: Like other eukaryotes, fungal cells contain membrane-bound nuclei with chromosomes that contain DNA with noncoding regions called introns and coding regions called exons. Fungi have membrane-bound cytoplasmic organelles such as mitochondria, sterol-containing membranes, and ribosomes of the 80S type.[12] They have a characteristic range of soluble carbohydrates and storage compounds, including sugar alcohols (e.g., mannitol), disaccharides, (e.g., trehalose), and polysaccharides (e.g., glycogen, which is also found in animals[13]).
- With animals: Fungi lack chloroplasts and are heterotrophic organisms and so require preformed organic compounds as energy sources.[14]
- With plants: Fungi have a cell wall[15] and vacuoles.[16] They reproduce by both sexual and asexual means, and like basal plant groups (such as ferns and mosses) produce spores. Similar to mosses and algae, fungi typically have haploid nuclei.[17]
- With euglenoids and bacteria: Higher fungi, euglenoids, and some bacteria produce the amino acid L-lysine in specific biosynthesis steps, called the α-aminoadipate pathway.[18][19]
- The cells of most fungi grow as tubular, elongated, and thread-like (filamentous) structures and are called hyphae, which may contain multiple nuclei and extend at their tips. Each tip contains a set of aggregated vesicles—cellular structures consisting of proteins, lipids, and other organic molecules—called Spitzenkörper.[20] Both fungi and oomycetes grow as filamentous hyphal cells.[21] In contrast, similar-looking organisms, such as filamentous green algae, grow by repeated cell division within a chain of cells.[13]
- In common with some plant and animal species, more than 70 fungal species display the phenomenon of bioluminescence.[22]
Unique features:
- Some species grow as unicellular yeasts that reproduce by budding or binary fission. Dimorphic fungi can switch between a yeast phase and a hyphal phase in response to environmental conditions.[23]
- The fungal cell wall is composed of glucans and chitin; while the former compounds are also found in plants and the latter in the exoskeleton of arthropods,[24][25] fungi are the only organisms that combine these two structural molecules in their cell wall. Unlike cell walls in plants and the oomycetes, those in fungi do not contain cellulose.[26]
Most fungi lack an efficient system for long-distance transport of water and nutrients, such as the
xylem and
phloem in many plants. To overcome these limitations, some fungi, such as
Armillaria, form
rhizomorphs,
[27] that resemble and perform functions similar to the
roots of plants. Another characteristic shared with plants is a
biosynthetic pathway for producing
terpenes that uses
mevalonic acid and
pyrophosphate as
chemical building blocks.
[28] However, plants have an additional terpene pathway in their chloroplasts, a structure fungi do not have.
[29] Fungi produce several
secondary metabolites that are similar or identical in structure to those made by plants.
[28] Many of the plant and fungal enzymes that make these compounds differ from each other in
sequence and other characteristics, which indicates separate origins and evolution of these enzymes in the fungi and plants.
[28][30]
Diversity
Fungi have a worldwide distribution, and grow in a wide range of habitats, including extreme environments such as
deserts or areas with high salt concentrations
[31] or
ionizing radiation,
[32] as well as in
deep sea sediments.
[33] Some can survive the intense
UV and
cosmic radiation encountered during space travel.
[34] Most grow in terrestrial environments, though several species live partly or solely in aquatic habitats, such as the
chytrid fungus
Batrachochytrium dendrobatidis, a
parasite that has been responsible for a worldwide decline in
amphibian populations. This organism spends part of its life cycle as a motile
zoospore, enabling it to propel itself through water and enter its amphibian host.
[35] Other examples of aquatic fungi include those living in
hydrothermal areas of the ocean.
[36]
Around 100,000 species of fungi have been formally
described by
taxonomists,
[37] but the global biodiversity of the fungus kingdom is not fully understood.
[38] On the basis of observations of the ratio of the number of fungal species to the number of plant species in selected environments, the fungal kingdom has been estimated to contain about 1.5 million species.
[39] A recent (2011) estimate suggests there may be over 5 million species.
[40] In mycology, species have historically been distinguished by a variety of methods and concepts. Classification based on
morphological characteristics, such as the size and shape of spores or fruiting structures, has traditionally dominated fungal taxonomy.
[41] Species may also be distinguished by their
biochemical and
physiological characteristics, such as their ability to metabolize certain biochemicals, or their reaction to
chemical tests. The
biological species concept discriminates species based on their ability to
mate. The application of
molecular tools, such as
DNA sequencing and phylogenetic analysis, to study diversity has greatly enhanced the resolution and added robustness to estimates of
genetic diversity within various taxonomic groups.
[42]

Two types of edible fungi
Mycology
Mycology is the branch of
biology concerned with the systematic study of fungi, including their genetic and biochemical properties, their taxonomy, and their use to humans as a source of medicine, food, and
psychotropic substances consumed for religious purposes, as well as their dangers, such as poisoning or infection. The field of
phytopathology, the study of plant diseases, is closely related because many plant pathogens are fungi.
[43]

In 1729, Pier A. Micheli first published descriptions of fungi.
The use of fungi by humans dates back to prehistory;
Ötzi the Iceman, a well-preserved mummy of a 5,300-year-old
Neolithic man found frozen in the Austrian Alps, carried two species of
polypore mushrooms that may have been used as
tinder (
Fomes fomentarius), or for medicinal purposes (
Piptoporus betulinus).
[44] Ancient peoples have used fungi as food sources–often unknowingly–for millennia, in the preparation of leavened bread and fermented juices. Some of the oldest written records contain references to the destruction of crops that were probably caused by pathogenic fungi.
[45]
History
Mycology is a relatively new science that became systematic after the development of the
microscope in the 16th century. Although fungal spores were first observed by
Giambattista della Porta in 1588, the seminal work in the development of mycology is considered to be the publication of
Pier Antonio Micheli's 1729 work
Nova plantarum genera.
[46] Micheli not only observed spores but also showed that, under the proper conditions, they could be induced into growing into the same species of fungi from which they originated.
[47] Extending the use of the
binomial system of nomenclature introduced by
Carl Linnaeus in his
Species plantarum (1753), the Dutch
Christian Hendrik Persoon (1761–1836) established the first classification of mushrooms with such skill so as to be considered a founder of modern mycology. Later,
Elias Magnus Fries (1794–1878) further elaborated the
classification of fungi, using spore color and various microscopic characteristics, methods still used by taxonomists today. Other notable early contributors to mycology in the 17th–19th and early 20th centuries include
Miles Joseph Berkeley,
August Carl Joseph Corda,
Anton de Bary, the brothers
Louis René and
Charles Tulasne,
Arthur H. R. Buller,
Curtis G. Lloyd, and
Pier Andrea Saccardo. The 20th century has seen a modernization of mycology that has come from advances in
biochemistry,
genetics,
molecular biology, and
biotechnology. The use of
DNA sequencing technologies and phylogenetic analysis has provided new insights into fungal relationships and
biodiversity, and has challenged traditional morphology-based groupings in fungal
taxonomy.
[48]
Morphology
Microscopic structures
Most fungi grow as
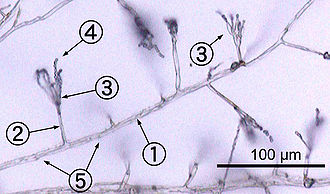
hyphae, which are cylindrical, thread-like structures 2–10
µm in diameter and up to several centimeters in length. Hyphae grow at their tips (apices); new hyphae are typically formed by emergence of new tips along existing hyphae by a process called
branching, or occasionally growing hyphal tips fork, giving rise to two parallel-growing hyphae.
[49] The combination of apical growth and branching/forking leads to the development of a
mycelium, an interconnected network of hyphae.
[23] Hyphae can be either
septate or
coenocytic. Septate hyphae are divided into compartments separated by cross walls (internal cell walls, called septa, that are formed at
right angles to the cell wall giving the hypha its shape), with each compartment containing one or more nuclei; coenocytic hyphae are not compartmentalized.
[50] Septa have
pores that allow
cytoplasm,
organelles, and sometimes nuclei to pass through; an example is the dolipore septum in fungi of the phylum Basidiomycota.
[51] Coenocytic hyphae are in essence
multinucleate supercells.
[52]
Many species have developed specialized hyphal structures for nutrient uptake from living hosts; examples include
haustoria in plant-parasitic species of most fungal phyla, and
arbuscules of several
mycorrhizal fungi, which penetrate into the host cells to consume nutrients.
[53]
Although fungi are
opisthokonts—a grouping of evolutionarily related organisms broadly characterized by a single posterior
flagellum—all phyla except for the
chytrids have lost their posterior flagella.
[54] Fungi are unusual among the eukaryotes in having a cell wall that, in addition to
glucans (e.g., β-1,3-glucan) and other typical components, also contains the
biopolymer chitin.
[55]
Macroscopic structures
Fungal mycelia can become visible to the naked eye, for example, on various surfaces and
substrates, such as damp walls and spoiled food, where they are commonly called
molds. Mycelia grown on solid
agar media in laboratory
petri dishes are usually referred to as
colonies. These colonies can exhibit growth shapes and colors (due to spores or
pigmentation) that can be used as diagnostic features in the identification of species or groups.
[56] Some individual fungal colonies can reach extraordinary dimensions and ages as in the case of a
clonal colony of
Armillaria solidipes, which extends over an area of more than 900
ha (3.5 square miles), with an estimated age of nearly 9,000 years.
[57]
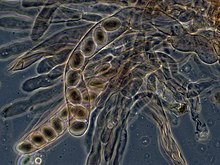
The
apothecium—a specialized structure important in
sexual reproduction in the ascomycetes—is a cup-shaped fruit body that holds the
hymenium, a layer of tissue containing the spore-bearing cells.
[58] The fruit bodies of the basidiomycetes (
basidiocarps) and some ascomycetes can sometimes grow very large, and many are well known as
mushrooms.
Growth and physiology

Mold growth covering a decaying
peach. The frames were taken approximately 12 hours apart over a period of six days.
The growth of fungi as hyphae on or in solid substrates or as single cells in aquatic environments is adapted for the efficient extraction of nutrients, because these growth forms have high
surface area to volume ratios.
[59] Hyphae are specifically adapted for growth on solid surfaces, and to invade
substrates and tissues.
[60] They can exert large penetrative mechanical forces; for example, the
plant pathogen Magnaporthe grisea forms a structure called an
appressorium that evolved to puncture plant tissues.
[61] The pressure generated by the appressorium, directed against the plant
epidermis, can exceed 8
megapascals (1,200 psi).
[61] The filamentous fungus
Paecilomyces lilacinus uses a similar structure to penetrate the eggs of
nematodes.
[62]
The mechanical pressure exerted by the appressorium is generated from physiological processes that increase intracellular
turgor by producing
osmolytes such as
glycerol.
[63] Adaptations such as these are complemented by
hydrolytic enzymes secreted into the environment to digest large organic molecules—such as
polysaccharides,
proteins, and
lipids—into smaller molecules that may then be absorbed as nutrients.
[64][65][66] The vast majority of filamentous fungi grow in a polar fashion—i.e., by extension into one direction—by elongation at the tip (apex) of the hypha.
[67] Other forms of fungal growth include intercalary extension (longitudinal expansion of hyphal compartments that are below the apex) as in the case of some
endophytic fungi,
[68] or growth by volume expansion during the development of mushroom
stipes and other large organs.
[69] Growth of fungi as
multicellular structures consisting of
somatic and reproductive cells—a feature independently evolved in animals and plants
[70]—has several functions, including the development of fruit bodies for dissemination of sexual spores (see above) and
biofilms for substrate colonization and
intercellular communication.
[71]
The fungi are traditionally considered
heterotrophs, organisms that rely solely on
carbon fixed by other organisms for
metabolism. Fungi have
evolved a high degree of metabolic versatility that allows them to use a diverse range of organic substrates for growth, including simple compounds such as
nitrate,
ammonia,
acetate, or
ethanol.
[72][73] In some species the pigment
melanin may play a role in extracting energy from
ionizing radiation, such as
gamma radiation. This form of
"radiotrophic" growth has been described for only a few species, the effects on growth rates are small, and the underlying
biophysical and biochemical processes are not well known.
[32] This process might bear similarity to
CO2 fixation via
visible light, but instead uses ionizing radiation as a source of energy.
[74]
Reproduction
Fungal reproduction is complex, reflecting the differences in lifestyles and genetic makeup within this diverse kingdom of organisms.
[75] It is estimated that a third of all fungi reproduce using more than one method of propagation; for example, reproduction may occur in two well-differentiated stages within the
life cycle of a species, the
teleomorph and the
anamorph.
[76] Environmental conditions trigger genetically determined developmental states that lead to the creation of specialized structures for sexual or asexual reproduction. These structures aid reproduction by efficiently dispersing spores or spore-containing
propagules.
Asexual reproduction
Asexual reproduction occurs via vegetative spores (
conidia) or through
mycelial fragmentation. Mycelial fragmentation occurs when a fungal mycelium separates into pieces, and each component grows into a separate mycelium. Mycelial fragmentation and vegatative spores maintain
clonal populations adapted to a specific
niche, and allow more rapid dispersal than sexual reproduction.
[77] The "Fungi imperfecti" (fungi lacking the perfect or sexual stage) or
Deuteromycota comprise all the species that lack an observable sexual cycle.
[78]
Sexual reproduction
Sexual reproduction with
meiosis exists in all fungal phyla except
Glomeromycota.
[79] It differs in many aspects from sexual reproduction in animals or plants. Differences also exist between fungal groups and can be used to discriminate species by morphological differences in sexual structures and reproductive strategies.
[80][81] Mating experiments between fungal isolates may identify species on the basis of biological species concepts.
[81] The major fungal groupings have initially been delineated based on the morphology of their sexual structures and spores; for example, the spore-containing structures,
asci and
basidia, can be used in the identification of ascomycetes and basidiomycetes, respectively. Some species may allow mating only between individuals of opposite
mating type, whereas others can mate and sexually reproduce with any other individual or itself. Species of the former
mating system are called
heterothallic, and of the latter
homothallic.
[82]
Most fungi have both a
haploid and a
diploid stage in their life cycles. In sexually reproducing fungi, compatible individuals may combine by fusing their hyphae together into an interconnected network; this process,
anastomosis, is required for the initiation of the sexual cycle. Ascomycetes and basidiomycetes go through a
dikaryotic stage, in which the nuclei inherited from the two parents do not combine immediately after cell fusion, but remain separate in the hyphal cells (see
heterokaryosis).
[83]
In ascomycetes, dikaryotic hyphae of the
hymenium (the spore-bearing tissue layer) form a characteristic
hook at the hyphal septum. During
cell division, formation of the hook ensures proper distribution of the newly divided nuclei into the apical and basal hyphal compartments. An ascus (plural
asci) is then formed, in which
karyogamy (nuclear fusion) occurs. Asci are embedded in an
ascocarp, or fruiting body. Karyogamy in the asci is followed immediately by meiosis and the production of
ascospores. After dispersal, the ascospores may germinate and form a new haploid mycelium.
[84]
Sexual reproduction in basidiomycetes is similar to that of the ascomycetes. Compatible haploid hyphae fuse to produce a dikaryotic mycelium. However, the dikaryotic phase is more extensive in the basidiomycetes, often also present in the vegetatively growing mycelium. A specialized anatomical structure, called a
clamp connection, is formed at each hyphal septum. As with the structurally similar hook in the ascomycetes, the clamp connection in the basidiomycetes is required for controlled transfer of nuclei during cell division, to maintain the dikaryotic stage with two genetically different nuclei in each hyphal compartment.
[85] A
basidiocarp is formed in which club-like structures known as
basidia generate haploid
basidiospores after karyogamy and meiosis.
[86] The most commonly known basidiocarps are mushrooms, but they may also take other forms (see
Morphology section).
In glomeromycetes (formerly zygomycetes), haploid hyphae of two individuals fuse, forming a
gametangium, a specialized cell structure that becomes a fertile
gamete-producing cell. The gametangium develops into a
zygospore, a thick-walled spore formed by the union of gametes. When the zygospore germinates, it undergoes
meiosis, generating new haploid hyphae, which may then form asexual
sporangiospores. These sporangiospores allow the fungus to rapidly disperse and germinate into new genetically identical haploid fungal mycelia.
[87]
Spore dispersal
Both asexual and sexual spores or sporangiospores are often actively dispersed by forcible ejection from their reproductive structures. This ejection ensures exit of the spores from the reproductive structures as well as traveling through the air over long distances.
Specialized mechanical and physiological mechanisms, as well as spore surface structures (such as
hydrophobins), enable efficient spore ejection.
[88] For example, the structure of the
spore-bearing cells in some ascomycete species is such that the buildup of
substances affecting cell volume and fluid balance enables the explosive discharge of spores into the air.
[89] The forcible discharge of single spores termed
ballistospores involves formation of a small drop of water (Buller's drop), which upon contact with the spore leads to its projectile release with an initial acceleration of more than 10,000
g;
[90] the net result is that the spore is ejected 0.01–0.02 cm, sufficient distance for it to fall through the gills or pores into the air below.
[91] Other fungi, like the
puffballs, rely on alternative mechanisms for spore release, such as external mechanical forces. The
bird's nest fungi use the force of falling water drops to liberate the spores from cup-shaped fruiting bodies.
[92] Another strategy is seen in the
stinkhorns, a group of fungi with lively colors and putrid odor that attract insects to disperse their spores.
[93]
Other sexual processes
Besides regular sexual reproduction with meiosis, certain fungi, such as those in the genera
Penicillium and
Aspergillus, may exchange genetic material via
parasexual processes, initiated by anastomosis between hyphae and
plasmogamy of fungal cells.
[94] The frequency and relative importance of parasexual events is unclear and may be lower than other sexual processes. It is known to play a role in intraspecific hybridization
[95] and is likely required for hybridization between species, which has been associated with major events in fungal evolution.
[96]
Evolution
In contrast to
plants and
animals, the early fossil record of the fungi is meager. Factors that likely contribute to the under-representation of fungal species among fossils include the nature of fungal
fruiting bodies, which are soft, fleshy, and easily degradable tissues and the microscopic dimensions of most fungal structures, which therefore are not readily evident. Fungal fossils are difficult to distinguish from those of other microbes, and are most easily identified when they resemble
extant fungi.
[97] Often recovered from a
permineralized plant or animal host, these samples are typically studied by making thin-section preparations that can be examined with
light microscopy or
transmission electron microscopy.
[98] Researchers study
compression fossils by dissolving the surrounding matrix with acid and then using light or
scanning electron microscopy to examine surface details.
[99]
The earliest fossils possessing features typical of fungi date to the
Proterozoic eon, some
1,430 million years ago (
Ma); these multicellular
benthic organisms had filamentous structures with septa, and were capable of
anastomosis.
[100] More recent studies (2009) estimate the arrival of fungal organisms at about 760–1060 Ma on the basis of comparisons of the rate of evolution in closely related groups.
[101] For much of the
Paleozoic Era (542–251 Ma), the fungi appear to have been aquatic and consisted of organisms similar to the extant
chytrids in having flagellum-bearing spores.
[102] The evolutionary adaptation from an aquatic to a terrestrial lifestyle necessitated a diversification of ecological strategies for obtaining nutrients, including
parasitism,
saprobism, and the development of
mutualistic relationships such as
mycorrhiza and lichenization.
[103] Recent (2009) studies suggest that the ancestral ecological state of the
Ascomycota was saprobism, and that independent
lichenization events have occurred multiple times.
[104]
It is presumed that the fungi colonized the land during the
Cambrian (542–488.3 Ma), long before land plants.
[105] Fossilized hyphae and spores recovered from the
Ordovician of Wisconsin (460 Ma) resemble modern-day
Glomerales, and existed at a time when the land flora likely consisted of only non-vascular
bryophyte-like plants.
[106] Prototaxites, which was probably a fungus or lichen, would have been the tallest organism of the late
Silurian. Fungal fossils do not become common and uncontroversial until the early
Devonian (416–359.2 Ma), when they occur abundantly in the
Rhynie chert, mostly as
Zygomycota and
Chytridiomycota.
[105][107][108] At about this same time, approximately 400 Ma, the Ascomycota and Basidiomycota diverged,
[109] and all modern
classes of fungi were present by the Late
Carboniferous (
Pennsylvanian, 318.1–299 Ma).
[110]
Lichen-like fossils have been found in the
Doushantuo Formation in southern China dating back to 635–551 Ma.
[111] Lichens formed a component of the early terrestrial ecosystems, and the estimated age of the oldest terrestrial lichen fossil is 400 Ma;
[112] this date corresponds to the age of the oldest known
sporocarp fossil, a
Paleopyrenomycites species found in the Rhynie Chert.
[113] The oldest fossil with microscopic features resembling modern-day basidiomycetes is
Palaeoancistrus, found permineralized with a
fern from the Pennsylvanian.
[114] Rare in the fossil record are the Homobasidiomycetes (a
taxon roughly equivalent to the mushroom-producing species of the
Agaricomycetes). Two
amber-preserved specimens provide evidence that the earliest known mushroom-forming fungi (the extinct species
Archaeomarasmius leggetti) appeared during the late
Cretaceous, 90 Ma.
[115][116]
Some time after the
Permian–Triassic extinction event (251.4 Ma), a fungal spike (originally thought to be an extraordinary abundance of fungal spores in
sediments) formed, suggesting that fungi were the dominant life form at this time, representing nearly 100% of the available
fossil record for this period.
[117] However, the relative proportion of fungal spores relative to spores formed by
algal species is difficult to assess,
[118] the spike did not appear worldwide,
[119][120] and in many places it did not fall on the Permian–Triassic boundary.
[121]
Taxonomy
Although commonly included in botany curricula and textbooks, fungi are more closely related to
animals than to plants and are placed with the animals in the
monophyletic group of
opisthokonts.
[122] Analyses using
molecular phylogenetics support a
monophyletic origin of the Fungi.
[42] The
taxonomy of the Fungi is in a state of constant flux, especially due to recent research based on DNA comparisons. These current phylogenetic analyses often overturn classifications based on older and sometimes less discriminative methods based on morphological features and biological species concepts obtained from experimental
matings.
[123]
There is no unique generally accepted system at the higher taxonomic levels and there are frequent name changes at every level, from species upwards. Efforts among researchers are now underway to establish and encourage usage of a unified and more consistent
nomenclature.
[42][124] Fungal species can also have multiple scientific names depending on their life cycle and mode (sexual or asexual) of reproduction. Web sites such as
Index Fungorum and
ITIS list current names of fungal species (with cross-references to older synonyms).
The 2007 classification of Kingdom Fungi is the result of a large-scale collaborative research effort involving dozens of mycologists and other scientists working on fungal taxonomy.
[42] It recognizes seven
phyla, two of which—the Ascomycota and the Basidiomycota—are contained within a branch representing
subkingdom Dikarya. The above
cladogram depicts the major fungal
taxa and their relationship to opisthokont and unikont organisms. The lengths of the branches in this tree are not proportional to
evolutionary distances.
Taxonomic groups
The major
phyla (sometimes called divisions) of fungi have been classified mainly on the basis of characteristics of their sexual
reproductive structures. Currently, seven phyla are proposed: Microsporidia, Chytridiomycota, Blastocladiomycota, Neocallimastigomycota, Glomeromycota, Ascomycota, and Basidiomycota.
[42]
Phylogenetic analysis has demonstrated that the
Microsporidia, unicellular parasites of animals and protists, are fairly recent and highly derived
endobiotic fungi (living within the tissue of another species).
[102][125] One 2006 study concludes that the Microsporidia are a sister group to the true fungi; that is, they are each other's closest evolutionary relative.
[126] Hibbett and colleagues suggest that this analysis does not clash with their classification of the Fungi, and although the Microsporidia are elevated to phylum status, it is acknowledged that further analysis is required to clarify evolutionary relationships within this group.
[42]
The
Chytridiomycota are commonly known as chytrids. These fungi are distributed worldwide. Chytrids produce
zoospores that are capable of active movement through aqueous phases with a single
flagellum, leading early
taxonomists to classify them as
protists.
Molecular phylogenies, inferred from
rRNA sequences in
ribosomes, suggest that the Chytrids are a
basal group divergent from the other fungal phyla, consisting of four major
clades with suggestive evidence for
paraphyly or possibly
polyphyly.
[127]
The
Blastocladiomycota were previously considered a taxonomic clade within the Chytridiomycota. Recent molecular data and
ultrastructural characteristics, however, place the Blastocladiomycota as a sister clade to the Zygomycota, Glomeromycota, and Dikarya (Ascomycota and Basidiomycota). The blastocladiomycetes are
saprotrophs, feeding on decomposing organic matter, and they are parasites of all eukaryotic groups. Unlike their close relatives, the chytrids, most of which exhibit
zygotic meiosis, the blastocladiomycetes undergo
sporic meiosis.
[102]
The
Neocallimastigomycota were earlier placed in the phylum Chytridomycota. Members of this small phylum are
anaerobic organisms, living in the digestive system of larger herbivorous mammals and in other terrestrial and aquatic environments enriched in cellulose (e.g., domestic waste landfill sites).
[128] They lack
mitochondria but contain
hydrogenosomes of mitochondrial origin. As the related chrytrids, neocallimastigomycetes form zoospores that are posteriorly uniflagellate or polyflagellate.
[42]
Members of the
Glomeromycota form
arbuscular mycorrhizae, a form of
symbiosis wherein fungal hyphae invade plant root cells and both species benefit from the resulting increased supply of nutrients. All known Glomeromycota species reproduce asexually.
[79] The symbiotic association between the Glomeromycota and plants is ancient, with evidence dating to 400 million years ago.
[129] Formerly part of the
Zygomycota (commonly known as 'sugar' and 'pin' molds), the Glomeromycota were elevated to phylum status in 2001 and now replace the older phylum Zygomycota.
[130] Fungi that were placed in the Zygomycota are now being reassigned to the Glomeromycota, or the subphyla
incertae sedis Mucoromycotina,
Kickxellomycotina, the
Zoopagomycotina and the
Entomophthoromycotina.
[42] Some well-known examples of fungi formerly in the Zygomycota include black bread mold (
Rhizopus stolonifer), and
Pilobolus species, capable of ejecting
spores several meters through the air.
[131] Medically relevant genera include
Mucor,
Rhizomucor, and
Rhizopus.

Diagram of an
apothecium (the typical cup-like reproductive structure of Ascomycetes) showing sterile tissues as well as developing and mature asci.
The
Ascomycota, commonly known as sac fungi or ascomycetes, constitute the largest taxonomic group within the Eumycota.
[41] These fungi form meiotic spores called
ascospores, which are enclosed in a special sac-like structure called an
ascus. This phylum includes
morels, a few
mushrooms and
truffles, unicellular
yeasts (e.g., of the genera
Saccharomyces,
Kluyveromyces,
Pichia, and
Candida), and many filamentous fungi living as saprotrophs, parasites, and mutualistic symbionts. Prominent and important genera of filamentous ascomycetes include
Aspergillus,
Penicillium,
Fusarium, and
Claviceps. Many ascomycete species have only been observed undergoing asexual reproduction (called
anamorphic species), but analysis of molecular data has often been able to identify their closest
teleomorphs in the Ascomycota.
[132] Because the products of meiosis are retained within the sac-like ascus, ascomycetes have been used for elucidating principles of genetics and heredity (e.g.,
Neurospora crassa).
[133]
Members of the
Basidiomycota, commonly known as the club fungi or basidiomycetes, produce meiospores called
basidiospores on club-like stalks called
basidia. Most common mushrooms belong to this group, as well as
rust and
smut fungi, which are major pathogens of grains. Other important basidiomycetes include the
maize pathogen
Ustilago maydis,
[134] human
commensal species of the genus
Malassezia,
[135] and the
opportunistic human pathogen,
Cryptococcus neoformans.
[136]
Fungus-like organisms
Because of similarities in morphology and lifestyle, the
slime molds (
mycetozoans,
plasmodiophorids,
acrasids,
Fonticula and
labyrinthulids, now in
Amoebozoa,
Rhizaria,
Excavata,
Opisthokonta and
Stramenopiles, respectively), water molds (
oomycetes) and
hyphochytrids (both
Stramenopiles) were formerly classified in the kingdom Fungi, in groups like
Mastigomycotina,
Gymnomycota and
Phycomycetes. The slime molds were studied also as
protozoans, leading to a
ambiregnal, duplicated taxonomy.
Unlike true fungi, the
cell walls of oomycetes contain
cellulose and lack
chitin. Hyphochytrids have both chitin and cellulose. Slime molds lack a cell wall during the assimilative phase (except labyrinthulids, which have a wall of scales), and ingest nutrients by ingestion (
phagocytosis, except labyrinthulids) rather than absorption (
osmotrophy, as fungi, labyrinthulids, oomycetes and hyphochytrids). Neither water molds nor slime molds are closely related to the true fungi, and, therefore,
taxonomists no longer group them in the kingdom Fungi. Nonetheless, studies of the oomycetes and myxomycetes are still often included in
mycology textbooks and primary research literature.
[137]
The
Eccrinales and
Amoebidiales are
opisthokont protists, previously thought to be zygomycete fungi. Other groups now in
Opisthokonta (e.g.,
Corallochytrium,
Ichthyosporea) were also at given time classified as fungi. The genus
Blastocystis, now in
Stramenopiles, was originally classified as a yeast.
Ellobiopsis, now in
Alveolata, was considered a chytrid. The
bacteria were also included in fungi in some classifications, as the group Schizomycetes.
The
Rozellida clade, including the "ex-chytrid"
Rozella, is a genetically disparate group known mostly from environmental DNA sequences that is a sister group to fungi. Members of the group that have been isolated lack the chitinous cell wall that is characteristic of fungi.
The
nucleariids, protists currently grouped in the
Choanozoa (
Opisthokonta), may be the next sister group to the eumycete clade, and as such could be included in an expanded fungal kingdom.
[138]
Ecology

A pin mold decomposing a peach
Although often inconspicuous, fungi occur in every environment on
Earth and play very important roles in most
ecosystems. Along with bacteria, fungi are the major
decomposers in most terrestrial (and some aquatic) ecosystems, and therefore play a critical role in
biogeochemical cycles[139] and in many
food webs. As decomposers, they play an essential role in
nutrient cycling, especially as
saprotrophs and
symbionts, degrading
organic matter to inorganic molecules, which can then re-enter anabolic metabolic pathways in plants or other organisms.
[140][141]
Symbiosis
Many fungi have important
symbiotic relationships with organisms from most if not all
Kingdoms.
[142][143][144] These interactions can be
mutualistic or antagonistic in nature, or in the case of
commensal fungi are of no apparent benefit or detriment to the host.
[145][146][147]
With plants
Mycorrhizal symbiosis between
plants and fungi is one of the most well-known plant–fungus associations and is of significant importance for plant growth and persistence in many ecosystems; over 90% of all plant species engage in mycorrhizal relationships with fungi and are dependent upon this relationship for survival.
[148]
The mycorrhizal symbiosis is ancient, dating to at least 400 million years ago.
[129] It often increases the plant's uptake of inorganic compounds, such as
nitrate and
phosphate from soils having low concentrations of these key plant nutrients.
[140][149] The fungal partners may also mediate plant-to-plant transfer of carbohydrates and other nutrients. Such mycorrhizal communities are called "common mycorrhizal networks".
[150] A special case of mycorrhiza is
myco-heterotrophy, whereby the plant parasitizes the fungus, obtaining all of its nutrients from its fungal symbiont.
[151] Some fungal species inhabit the tissues inside roots, stems, and leaves, in which case they are called endophytes.
[152] Similar to mycorrhiza, endophytic colonization by fungi may benefit both symbionts; for example, endophytes of grasses impart to their host increased resistance to herbivores and other environmental stresses and receive food and shelter from the plant in return.
[153]
With algae and cyanobacteria
Lichens are a symbiotic relationship between fungi and
algae or
cyanobacteria. The algae partner in the relationship is referred to in lichen terminology as a "photobiont". The fungi part of the relationship are composed mostly of various species of
ascomycetes and a few
basidiomycetes.
[154] Lichens occur in every ecosystem on all continents, play a key role in
soil formation and the initiation of
biological succession,
[155] and are the dominating life forms in extreme environments, including
polar,
alpine, and
semiarid desert regions.
[156] They are able to grow on inhospitable surfaces, including bare soil, rocks,
tree bark, wood, shells, barnacles and leaves.
[157] As in
mycorrhizas, the photobiont provides sugars and other carbohydrates via
photosynthesis to the fungus, while the fungus provides minerals and water to the photobiont. The functions of both symbiotic organisms are so closely intertwined that they function almost as a single organism; in most cases the resulting organism differs greatly from the individual components. Lichenization is a common mode of nutrition for fungi; around 20% of fungi—between 17,500 and 20,000 described species—are lichenized.
[158] Characteristics common to most lichens include obtaining
organic carbon by photosynthesis, slow growth, small size, long life, long-lasting (seasonal)
vegetative reproductive structures, mineral nutrition obtained largely from airborne sources, and greater tolerance of
desiccation than most other photosynthetic organisms in the same habitat.
[159]
With insects
Many insects also engage in
mutualistic relationships with fungi. Several groups of ants cultivate fungi in the order
Agaricales as their primary food source, while
ambrosia beetles cultivate various species of fungi in the bark of trees that they infest.
[160] Likewise, females of several
wood wasp species (genus
Sirex) inject their eggs together with spores of the wood-rotting fungus
Amylostereum areolatum into the
sapwood of
pine trees; the growth of the fungus provides ideal nutritional conditions for the development of the wasp larvae.
[161] Termites on the African
savannah are also known to cultivate fungi,
[142] and yeasts of the genera
Candida and
Lachancea inhabit the
gut of a wide range of insects, including
neuropterans,
beetles, and
cockroaches; it is not known whether these fungi benefit their hosts.
[162] The larvae of many families of fungicolous flies, particularly those within the superfamily
Sciaroidea such as the
Mycetophilidae and some
Keroplatidae feed on fungal fruiting bodies and sterile
mycorrhizae.
[163]
As pathogens and parasites
Many fungi are
parasites on plants, animals (including humans), and other fungi. Serious pathogens of many cultivated plants causing extensive damage and losses to agriculture and forestry include the
rice blast fungus
Magnaporthe oryzae,
[164] tree pathogens such as
Ophiostoma ulmi and
Ophiostoma novo-ulmi causing
Dutch elm disease,
[165] and
Cryphonectria parasitica responsible for
chestnut blight,
[166] and plant pathogens in the genera
Fusarium,
Ustilago,
Alternaria, and
Cochliobolus.
[146] Some
carnivorous fungi, like
Paecilomyces lilacinus, are
predators of
nematodes, which they capture using an array of specialized structures such as constricting rings or adhesive nets.
[167]
Some fungi can cause serious diseases in humans, several of which may be fatal if untreated. These include
aspergilloses,
candidoses,
coccidioidomycosis,
cryptococcosis,
histoplasmosis,
mycetomas, and
paracoccidioidomycosis. Furthermore, persons with
immuno-deficiencies are particularly susceptible to disease by genera such as
Aspergillus,
Candida,
Cryptoccocus,
[147][168][169] Histoplasma,
[170] and
Pneumocystis.
[171] Other fungi can attack eyes, nails, hair, and especially skin, the so-called
dermatophytic and keratinophilic fungi, and cause local infections such as
ringworm and
athlete's foot.
[172] Fungal spores are also a cause of
allergies, and fungi from different taxonomic groups can evoke allergic reactions.
[173]
Mycotoxins
Mycotoxins are secondary metabolites (or
natural products), and research has established the existence of biochemical pathways solely for the purpose of producing mycotoxins and other natural products in fungi.
[28] Mycotoxins may provide
fitness benefits in terms of physiological adaptation, competition with other microbes and fungi, and protection from consumption (
fungivory).
[174][175] Many fungi produce
biologically active compounds, several of which are
toxic to animals or plants and are therefore called
mycotoxins. Of particular relevance to humans are mycotoxins produced by molds causing food spoilage, and poisonous mushrooms (see above). Particularly infamous are the lethal
amatoxins in some
Amanita mushrooms, and
ergot alkaloids, which have a long history of causing serious epidemics of
ergotism (St Anthony's Fire) in people consuming
rye or related
cereals contaminated with
sclerotia of the ergot fungus,
Claviceps purpurea.
[176] Other notable mycotoxins include the
aflatoxins, which are insidious
liver toxins and highly
carcinogenic metabolites produced by certain
Aspergillus species often growing in or on grains and nuts consumed by humans,
ochratoxins,
patulin, and
trichothecenes (e.g.,
T-2 mycotoxin) and
fumonisins, which have significant impact on human food supplies or animal
livestock.
[177]
Pathogenic mechanisms
Ustilago maydis is a pathogenic plant fungus that causes smut disease in maize and
teosinte. Plants have evolved efficient defense systems against pathogenic microbes such as
U. maydis. A rapid defense reaction after pathogen attack is the
oxidative burst where the plant produces
reactive oxygen species at the site of the attempted invasion.
U. maydis can respond to the oxidative burst with an oxidative stress response, regulated by the gene
YAP1. The response protects
U. maydis from the host defense, and is necessary for the pathogen’s virulence.
[178] Furthermore,
U. maydis has a well-established recombinational
DNA repair system which acts during mitosis and meiosis.
[179] The system may assist the pathogen in surviving DNA damage arising from the host plant’s oxidative defensive response to infection.
[180]
Cryptococcus neoformans is an encapsulated yeast that can live in both plants and animals.
C. neoformans usually infects the lungs, where it is phagocytosed by
alveolar macrophages.
[181] Some
C. neoformans can survive
inside macrophage, which appears to be the basis for
latency, disseminated disease, and resistance to antifungal agents. One mechanism by which
C. neoformans survives the hostile macrophage environment is by up-regulating the expression of genes involved in the oxidative stress response.
[181] Another mechanism involves
meiosis. The majority of
C. neoformans are mating "type a". Filaments of mating "type an" ordinarily have haploid nuclei, but they can become diploid (perhaps by endoduplication or by stimulated nuclear fusion) to form
blastospores. The diploid nuclei of blastospores can undergo meiosis, including recombination, to form haploid basidiospores that can be dispersed.
[182] This process is referred to as monokaryotic fruiting. this process requires a gene called
DMC1, which is a conserved homologue of genes
recA in bacteria and
RAD51 in eukaryotes, that mediates homologous chromosome pairing during meiosis and repair of DNA double-strand breaks. Thus,
C. neoformans can undergo a meiosis, monokaryotic fruiting, that promotes recombinational repair in the oxidative, DNA damaging environment of the host macrophage, and the repair capability may contribute to its virulence.
[180][182]
Human use
The human use of fungi for food preparation or preservation and other purposes is extensive and has a long history.
Mushroom farming and
mushroom gathering are large industries in many countries. The study of the historical uses and sociological impact of fungi is known as
ethnomycology. Because of the capacity of this group to produce an enormous range of
natural products with
antimicrobial or other biological activities, many species have long been used or are being developed for industrial
production of antibiotics, vitamins, and
anti-cancer and
cholesterol-lowering drugs. More recently, methods have been developed for
genetic engineering of fungi,
[183] enabling
metabolic engineering of fungal species. For example, genetic modification of yeast species
[184]—which are easy to grow at fast rates in large fermentation vessels—has opened up ways of
pharmaceutical production that are potentially more efficient than production by the original source organisms.
[185]
Therapeutic uses
Modern chemotherapeutics
Many species produce metabolites that are major sources of
pharmacologically active drugs. Particularly important are the antibiotics, including the
penicillins, a structurally related group of
β-lactam antibiotics that are synthesized from small
peptides. Although naturally occurring penicillins such as
penicillin G (produced by
Penicillium chrysogenum) have a relatively narrow spectrum of biological activity, a wide range of other penicillins can be produced by
chemical modification of the natural penicillins. Modern penicillins are
semisynthetic compounds, obtained initially from
fermentation cultures, but then structurally altered for specific desirable properties.
[186] Other antibiotics produced by fungi include:
ciclosporin, commonly used as an
immunosuppressant during
transplant surgery; and
fusidic acid, used to help control infection from
methicillin-resistant Staphylococcus aureus bacteria.
[187] Widespread use of antibiotics for the treatment of bacterial diseases, such as
tuberculosis,
syphilis,
leprosy, and others began in the early 20th century and continues to date. In nature, antibiotics of fungal or bacterial origin appear to play a dual role: at high concentrations they act as chemical defense against competition with other microorganisms in species-rich environments, such as the
rhizosphere, and at low concentrations as
quorum-sensing molecules for intra- or interspecies signaling.
[188] Other drugs produced by fungi include
griseofulvin isolated from
Penicillium griseofulvum, used to treat fungal infections,
[189] and
statins (
HMG-CoA reductase inhibitors), used to inhibit
cholesterol synthesis. Examples of statins found in fungi include
mevastatin from
Penicillium citrinum and
lovastatin from
Aspergillus terreus and the
oyster mushroom.
[190]
Traditional and folk medicine
Certain mushrooms enjoy usage as therapeutics in
folk medicines, such as
Traditional Chinese medicine. Notable medicinal mushrooms with a well-documented history of use include
Agaricus subrufescens,
[191][192] Ganoderma lucidum,
[193] and
Ophiocordyceps sinensis.
[194] Research has identified compounds produced by these and other fungi that have inhibitory biological effects against
viruses[195][196] and
cancer cells.
[191][197] Specific metabolites, such as
polysaccharide-K,
ergotamine, and
β-lactam antibiotics, are routinely used in clinical medicine. The
shiitake mushroom is a source of
lentinan, a clinical drug approved for use in cancer treatments in several countries, including
Japan.
[198][199] In
Europe and
Japan,
polysaccharide-K (brand name Krestin), a chemical derived from
Trametes versicolor, is an approved
adjuvant for cancer therapy.
[200]
Cultured foods
Baker's yeast or
Saccharomyces cerevisiae, a unicellular fungus, is used to make
bread and other wheat-based products, such as
pizza dough and
dumplings.
[201] Yeast species of the genus
Saccharomyces are also used to produce
alcoholic beverages through fermentation.
[202] Shoyu koji mold (
Aspergillus oryzae) is an essential ingredient in brewing
Shoyu (
soy sauce) and
sake, and the preparation of
miso,
[203] while
Rhizopus species are used for making
tempeh.
[204] Several of these fungi are
domesticated species that were
bred or selected according to their capacity to ferment food without producing harmful mycotoxins (see below), which are produced by very closely related
Aspergilli.
[205] Quorn, a
meat substitute, is made from
Fusarium venenatum.
[206]
Edible and poisonous species
Edible mushrooms are well-known examples of fungi. Many are commercially raised, but others must be harvested from the wild.
Agaricus bisporus, sold as button mushrooms when small or Portobello mushrooms when larger, is a commonly eaten species, used in salads, soups, and many other dishes. Many Asian fungi are commercially grown and have increased in popularity in the West. They are often available fresh in
grocery stores and markets, including straw mushrooms (
Volvariella volvacea), oyster mushrooms (
Pleurotus ostreatus), shiitakes (
Lentinula edodes), and
enokitake (
Flammulina spp.).
[207]
There are many more mushroom species that are
harvested from the wild for personal consumption or commercial sale.
Milk mushrooms,
morels,
chanterelles,
truffles,
black trumpets, and
porcini mushrooms (
Boletus edulis) (also known as king boletes) demand a high price on the market. They are often used in gourmet dishes.
[208]
Certain types of cheeses require inoculation of milk curds with fungal species that impart a unique flavor and texture to the cheese. Examples include the
blue color in cheeses such as
Stilton or
Roquefort, which are made by inoculation with
Penicillium roqueforti.
[209] Molds used in cheese production are non-toxic and are thus safe for human consumption; however, mycotoxins (e.g., aflatoxins,
roquefortine C, patulin, or others) may accumulate because of growth of other fungi during cheese ripening or storage.
[210]
Many mushroom species are
poisonous to humans, with toxicities ranging from slight digestive problems or
allergic reactions as well as
hallucinations to severe organ failures and death. Genera with mushrooms containing deadly toxins include
Conocybe,
Galerina,
Lepiota, and, the most infamous,
Amanita.
[211] The latter genus includes the destroying angel
(A. virosa) and the death cap
(A. phalloides), the most common cause of deadly mushroom poisoning.
[212] The false morel (
Gyromitra esculenta) is occasionally considered a delicacy when cooked, yet can be highly toxic when eaten raw.
[213] Tricholoma equestre was considered edible until it was implicated in serious poisonings causing
rhabdomyolysis.
[214] Fly agaric mushrooms (
Amanita muscaria) also cause occasional non-fatal poisonings, mostly as a result of ingestion for its
hallucinogenic properties. Historically, fly agaric was used by different peoples in Europe and Asia and its present usage for religious or
shamanic purposes is reported from some ethnic groups such as the
Koryak people of north-eastern
Siberia.
[215]
As it is difficult to accurately identify a safe mushroom without proper training and knowledge, it is often advised to assume that a wild mushroom is poisonous and not to consume it.
[216][217]
Pest control
In agriculture, fungi may be useful if they actively compete for nutrients and space with
pathogenic microorganisms such as bacteria or other fungi via the
competitive exclusion principle,
[218] or if they are
parasites of these pathogens. For example, certain species may be used to eliminate or suppress the growth of harmful plant pathogens, such as insects,
mites,
weeds,
nematodes, and other fungi that cause diseases of important
crop plants.
[219] This has generated strong interest in practical applications that use these fungi in the
biological control of these agricultural pests.
Entomopathogenic fungi can be used as
biopesticides, as they actively kill insects.
[220] Examples that have been used as
biological insecticides are
Beauveria bassiana,
Metarhizium spp,
Hirsutella spp,
Paecilomyces (
Isaria) spp, and
Lecanicillium lecanii.
[221][222] Endophytic fungi of grasses of the genus
Neotyphodium, such as
N. coenophialum, produce alkaloids that are toxic to a range of invertebrate and vertebrate
herbivores. These alkaloids protect grass plants from
herbivory, but several endophyte alkaloids can poison grazing animals, such as cattle and sheep.
[223] Infecting cultivars of
pasture or
forage grasses with
Neotyphodium endophytes is one approach being used in
grass breeding programs; the fungal strains are selected for producing only alkaloids that increase resistance to herbivores such as insects, while being non-toxic to livestock.
[224]
Bioremediation
Certain fungi, in particular "white rot" fungi, can degrade
insecticides,
herbicides,
pentachlorophenol,
creosote,
coal tars, and heavy fuels and turn them into
carbon dioxide, water, and basic elements.
[225] Fungi have been shown to
biomineralize uranium oxides, suggesting they may have application in the bioremediation of radioactively polluted sites.
[226][227][228]
Model organisms
Several pivotal discoveries in biology were made by researchers using fungi as
model organisms, that is, fungi that grow and sexually reproduce rapidly in the laboratory. For example, the
one gene-one enzyme hypothesis was formulated by scientists using the bread mold
Neurospora crassa to test their biochemical theories.
[229] Other important model fungi are
Aspergillus nidulans and the yeasts
Saccaromyces cerevisiae and
Schizosaccharomyces pombe, each of which with a long history of use to investigate issues in eukaryotic
cell biology and
genetics, such as
cell cycle regulation,
chromatin structure, and
gene regulation. Other fungal models have more recently emerged that each address specific biological questions relevant to
medicine,
plant pathology, and industrial uses; examples include
Candida albicans, a dimorphic, opportunistic human pathogen,
[230] Magnaporthe grisea, a plant pathogen,
[231] and
Pichia pastoris, a yeast widely used for eukaryotic
protein expression.
[232]
Others
Fungi are used extensively to produce industrial chemicals like
citric,
gluconic,
lactic, and
malic acids,
[233] and industrial enzymes, such as
lipases used in
biological detergents,
[234] cellulases used in making
cellulosic ethanol[235] and
stonewashed jeans,
[236] and
amylases,
[237] invertases,
proteases and
xylanases.
[238] Several species, most notably
Psilocybin mushrooms (colloquially known as
magic mushrooms), are ingested for their
psychedelic properties, both
recreationally and religiously.





















![(6aR,9R)-N-((2R,5S,10aS,10bS)-5-benzyl-10b-hydroxy-2-methyl-3,6-dioxooctahydro-2H-oxazolo[3,2-a] pyrrolo[2,1-c]pyrazin-2-yl)-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg] quinoline-9-carboxamide](http://upload.wikimedia.org/wikipedia/commons/thumb/9/94/Ergotamine3.png/220px-Ergotamine3.png)






