From Wikipedia, the free encyclopedia
Enthalpy ( listen)
listen) is a measurement of
energy in a
thermodynamic system.
It is the thermodynamic quantity equivalent to the total heat content
of a system. It is equal to the internal energy of the system plus the
product of pressure and volume.
[1]
More technically, it includes the
internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its
environment and establishing its volume and pressure.
[2]
Enthalpy is defined as a
state function
that depends only on the prevailing equilibrium state identified by the
system's internal energy, pressure, and volume. It is an
extensive quantity. The unit of measurement for enthalpy in the
International System of Units (SI) is the
joule, but other historical, conventional units are still in use, such as the
British thermal unit and the
calorie.
Enthalpy is the preferred expression of system energy changes in many
chemical, biological, and physical measurements at constant pressure,
because it simplifies the description of
energy transfer.
At constant pressure, the enthalpy change equals the energy transferred
from the environment through heating or work other than expansion work.
The total enthalpy,
H, of a system cannot be measured
directly. The same situation exists in classical mechanics: only a
change or difference in energy carries physical meaning. Enthalpy itself
is a thermodynamic potential, so in order to measure the enthalpy of a
system, we must refer to a defined reference point; therefore what we
measure is the change in enthalpy, Δ
H. The Δ
H is a positive change in
endothermic reactions, and negative in heat-releasing
exothermic processes.
For processes under constant pressure, Δ
H is equal to the change in the internal energy of the system, plus the
pressure-volume work that the system has done on its surroundings.
[3]
This means that the change in enthalpy under such conditions is the
heat absorbed (or released) by the material through a chemical reaction
or by external heat transfer. Enthalpies for chemical substances at
constant pressure assume
standard state: most commonly 1 bar pressure. Standard state does not, strictly speaking, specify a temperature (see
standard state), but expressions for enthalpy generally reference the standard heat of formation at 25 °C.
Enthalpy of
ideal gases and incompressible solids and liquids does not depend on pressure, unlike
entropy and
Gibbs energy.
Real materials at common temperatures and pressures usually closely
approximate this behavior, which greatly simplifies enthalpy calculation
and use in practical designs and analyses.
Origins
The word
enthalpy stems from the
Ancient Greek verb
enthalpein (
ἐνθάλπειν), which means "to warm in".
[4] It combines the
Classical Greek prefix
ἐν- en-, meaning "to put into", and the verb
θάλπειν thalpein, meaning "to heat". The word
enthalpy is often incorrectly attributed to
Benoît Paul Émile Clapeyron and
Rudolf Clausius through the 1850 publication of their
Clausius–Clapeyron relation. This misconception was popularized by the 1927 publication of
The Mollier Steam Tables and Diagrams. However, neither the concept, the word, nor the symbol for enthalpy existed until well after Clapeyron's death.
The earliest writings to contain the concept of enthalpy did not appear until 1875,
[5] when
Josiah Willard Gibbs introduced "a heat function for constant pressure". However, Gibbs did not use the word "enthalpy" in his writings.
[note 1]
The actual word first appears in the scientific literature in a 1909
publication by J. P. Dalton. According to that publication,
Heike Kamerlingh Onnes actually coined the word.
[6]
Over the years, scientists used many different symbols to denote enthalpy. In 1922 Alfred W. Porter proposed the symbol "
H" as a standard,
[7] thus finalizing the terminology still in use today.
Formal definition
The enthalpy of a homogeneous system is defined as
[8][9]

where
- H is the enthalpy of the system,
- U is the internal energy of the system,
- p is the pressure of the system,
- V is the volume of the system.
Enthalpy is an
extensive property.
This means that, for homogeneous systems, the enthalpy is proportional
to the size of the system. It is convenient to introduce the
specific enthalpy h =
H/m, where
m is the mass of the system, or the molar enthalpy
Hm =
H/n, where
n is the number of moles (
h and
Hm are
intensive properties). For inhomogeneous systems the enthalpy is the sum of the enthalpies of the composing subsystems:

where the label
k refers to the various subsystems. In case of continuously varying
p,
T or composition, the summation becomes an integral:

where
ρ is the density.
The enthalpy of homogeneous systems can be viewed as function
H(
S,
p) of the entropy
S and the pressure
p, and a differential relation for it can be derived as follows. We start from the
first law of thermodynamics for closed systems for an infinitesimal process:

Here,
δQ is a small amount of heat added to the system, and
δW a small amount of work performed by the system. In a homogeneous system only reversible processes can take place, so the
second law of thermodynamics gives
δQ = T dS, with
T the
absolute temperature of the system. Furthermore, if only
pV work is done,
δW = p dV. As a result,

Adding
d(
pV) to both sides of this expression gives

or

So

Other expressions
The above expression of
dH
in terms of entropy and pressure may be unfamiliar to some readers.
However, there are expressions in terms of more familiar variables such
as temperature and pressure:
[8]:88[10]

Here
Cp is the heat capacity at constant pressure and
α is the coefficient of (cubic) thermal expansion:

With this expression one can, in principle, determine the enthalpy if
Cp and
V are known as functions of
p and
T.
Note that for an
ideal gas,
αT = 1,
[note 2] so that

In a more general form, the first law describes the internal energy with additional terms involving the
chemical potential and the number of particles of various types. The differential statement for
dH then becomes

where
μi is the chemical potential per particle for an
i-type particle, and
Ni is the number of such particles. The last term can also be written as
μi dni (with
dni the number of moles of component
i added to the system and, in this case,
μi the molar chemical potential) or as
μi dmi (with
dmi the mass of component
i added to the system and, in this case,
μi the specific chemical potential).
Physical interpretation
The
U term can be interpreted as the energy required to create the system, and the
pV
term as the energy that would be required to "make room" for the system
if the pressure of the environment remained constant. When a system,
for example,
n moles of a gas of
volume V at
pressure p and
temperature T, is created or brought to its present state from
absolute zero, energy must be supplied equal to its internal energy
U plus
pV, where
pV is the
work done in pushing against the ambient (atmospheric) pressure.
In basic
physics and
statistical mechanics it may be more interesting to study the internal properties of the system and therefore the internal energy is used.
[11][12] In basic
chemistry, experiments are often conducted at constant
atmospheric pressure,
and the pressure-volume work represents an energy exchange with the
atmosphere that cannot be accessed or controlled, so that Δ
H is the expression chosen for the
heat of reaction.
For a
heat engine a change in its internal energy is the difference between the heat input and the
pressure-volume work
done by the working substance while a change in its enthalpy is the
difference between the heat input and the work done by the engine:
[13]

where the work W done by the engine is:

Relationship to heat
In order to discuss the relation between the enthalpy increase and heat supply, we return to the first law for closed systems:
dU = δQ − δW.
We apply it to the special case with a uniform pressure at the surface.
In this case the work term can be split into two contributions, the
so-called
pV work, given by
p dV (where here
p is the pressure at the surface,
dV is the increase of the volume of the system) and all other types of work
δW′, such as by a shaft or by electromagnetic interaction. So we write
δW = p dV + δW′. In this case the first law reads:

or

From this relation we see that the increase in enthalpy of a system is equal to the added
heat:

provided that the system is under
constant pressure (
dp = 0) and that the only work done by the system is expansion work (
δW' = 0).
[14]
Applications
In
thermodynamics, one can calculate enthalpy by determining the
requirements for creating a system from "nothingness"; the mechanical
work required,
pV, differs based upon the conditions that obtain during the creation of the
thermodynamic system.
Energy
must be supplied to remove particles from the surroundings to make
space for the creation of the system, assuming that the pressure
p remains constant; this is the
pV term. The supplied energy must also provide the change in internal energy,
U, which includes
activation energies,
ionization energies, mixing energies, vaporization energies, chemical
bond energies, and so forth. Together, these constitute the change in
the enthalpy
U +
pV. For systems at constant pressure, with no external work done other than the
pV work, the change in enthalpy is the heat received by the system.
For a simple system, with a constant number of particles, the
difference in enthalpy is the maximum amount of thermal energy derivable
from a thermodynamic process in which the pressure is held constant.
[15]
Heat of reaction
The total enthalpy of a system cannot be measured directly, the
enthalpy change of a
system is measured instead. Enthalpy change is defined by the following equation:

where
- ΔH is the "enthalpy change",
- Hf is the final enthalpy of the system (in a chemical reaction, the enthalpy of the products),
- Hi is the initial enthalpy of the system (in a chemical reaction, the enthalpy of the reactants).
For an
exothermic reaction at constant
pressure,
the system's change in enthalpy equals the energy released in the
reaction, including the energy retained in the system and lost through
expansion against its surroundings. In a similar manner, for an
endothermic reaction, the system's change in enthalpy is equal to the energy
absorbed in the reaction, including the energy
lost by the system and
gained
from compression from its surroundings. A relatively easy way to
determine whether or not a reaction is exothermic or endothermic is to
determine the sign of Δ
H. If Δ
H is positive, the reaction
is endothermic, that is heat is absorbed by the system due to the
products of the reaction having a greater enthalpy than the reactants.
On the other hand, if Δ
H is negative, the reaction is exothermic, that is the overall decrease in enthalpy is achieved by the generation of heat.
[16]
Specific enthalpy
The specific enthalpy of a uniform system is defined as
h =
H/m where
m is the mass of the system. The
SI unit for specific enthalpy is joule per kilogram. It can be expressed in other specific quantities by
h = u + pv, where
u is the specific
internal energy,
p is the pressure, and
v is specific volume, which is equal to
1/ρ, where
ρ is the density.
Enthalpy changes
An
enthalpy change describes the change in enthalpy observed in the
constituents of a thermodynamic system when undergoing a transformation
or chemical reaction. It is the difference between the enthalpy after
the process has completed, i.e. the enthalpy of the
products, and the initial enthalpy of the system, i.e. the reactants. These processes are reversible
[why?] and the enthalpy for the reverse process is the negative value of the forward change.
A common standard enthalpy change is the
enthalpy of formation,
which has been determined for a large number of substances. Enthalpy
changes are routinely measured and compiled in chemical and physical
reference works, such as the
CRC Handbook of Chemistry and Physics. The following is a selection of enthalpy changes commonly recognized in thermodynamics.
When used in these recognized terms the qualifier
change is usually dropped and the property is simply termed
enthalpy of 'process'.
Since these properties are often used as reference values it is very
common to quote them for a standardized set of environmental parameters,
or
standard conditions, including:
- A temperature of 25 °C or 298 K,
- A pressure of one atmosphere (1 atm or 101.325 kPa),
- A concentration of 1.0 M when the element or compound is present in solution,
- Elements or compounds in their normal physical states, i.e. standard state.
For such standardized values the name of the enthalpy is commonly prefixed with the term
standard, e.g.
standard enthalpy of formation.
Chemical properties:
- Enthalpy of reaction,
defined as the enthalpy change observed in a constituent of a
thermodynamic system when one mole of substance reacts completely.
- Enthalpy of formation,
defined as the enthalpy change observed in a constituent of a
thermodynamic system when one mole of a compound is formed from its
elementary antecedents.
- Enthalpy of combustion,
defined as the enthalpy change observed in a constituent of a
thermodynamic system when one mole of a substance burns completely with
oxygen.
- Enthalpy of hydrogenation,
defined as the enthalpy change observed in a constituent of a
thermodynamic system when one mole of an unsaturated compound reacts
completely with an excess of hydrogen to form a saturated compound.
- Enthalpy of atomization, defined as the enthalpy change required to atomize one mole of compound completely.
- Enthalpy of neutralization,
defined as the enthalpy change observed in a constituent of a
thermodynamic system when one mole of water is formed when an acid and a
base react.
- Standard Enthalpy of solution,
defined as the enthalpy change observed in a constituent of a
thermodynamic system when one mole of a solute is dissolved completely
in an excess of solvent, so that the solution is at infinite dilution.
- Standard enthalpy of Denaturation (biochemistry), defined as the enthalpy change required to denature one mole of compound.
- Enthalpy of hydration,
defined as the enthalpy change observed when one mole of gaseous ions
are completely dissolved in water forming one mole of aqueous ions.
Physical properties:
- Enthalpy of fusion,
defined as the enthalpy change required to completely change the state
of one mole of substance between solid and liquid states.
- Enthalpy of vaporization,
defined as the enthalpy change required to completely change the state
of one mole of substance between liquid and gaseous states.
- Enthalpy of sublimation,
defined as the enthalpy change required to completely change the state
of one mole of substance between solid and gaseous states.
- Lattice enthalpy,
defined as the energy required to separate one mole of an ionic
compound into separated gaseous ions to an infinite distance apart
(meaning no force of attraction).
- Enthalpy of mixing, defined as the enthalpy change upon mixing of two (non-reacting) chemical substances.
Open systems
In
thermodynamic open systems,
matter may flow in and out of the system boundaries. The first law of
thermodynamics for open systems states: The increase in the internal
energy of a system is equal to the amount of energy added to the system
by matter flowing in and by heating, minus the amount lost by matter
flowing out and in the form of work done by the system:

where
Uin is the average internal energy entering the system, and
Uout is the average internal energy leaving the system.
During
steady, continuous
operation, an energy balance applied to an open system equates shaft
work performed by the system to heat added plus net enthalpy added
The region of space enclosed by the boundaries of the open system is usually called a
control volume,
and it may or may not correspond to physical walls. If we choose the
shape of the control volume such that all flow in or out occurs
perpendicular to its surface, then the flow of matter into the system
performs work as if it were a piston of fluid pushing mass into the
system, and the system performs work on the flow of matter out as if it
were driving a piston of fluid. There are then two types of work
performed:
flow work described above, which is performed on the fluid (this is also often called
pV work), and
shaft work, which may be performed on some mechanical device.
These two types of work are expressed in the equation

Substitution into the equation above for the control volume (cv) yields:

The definition of enthalpy,
H, permits us to use this
thermodynamic potential to account for both internal energy and
pV work in fluids for open systems:

If we allow also the system boundary to move (e.g. due to moving
pistons), we get a rather general form of the first law for open
systems.
[17] In terms of time derivatives it reads:

with sums over the various places
k where heat is supplied, matter flows into the system, and boundaries are moving. The
Ḣk terms represent enthalpy flows, which can be written as

with
ṁk the mass flow and
ṅk the molar flow at position
k respectively. The term
dVk/dt represents the rate of change of the system volume at position
k that results in
pV power done by the system. The parameter
P
represents all other forms of power done by the system such as shaft
power, but it can also be e.g. electric power produced by an electrical
power plant.
Note that the previous expression holds true only if the kinetic energy flow rate is conserved between system inlet and outlet.
[clarification needed] Otherwise, it has to be included in the enthalpy balance. During
steady-state operation of a device (
see turbine, pump, and engine), the average
dU/dt may be set equal to zero. This yields a useful expression for the average
power generation for these devices in the absence of chemical reactions:

where the
angle brackets
denote time averages. The technical importance of the enthalpy is
directly related to its presence in the first law for open systems, as
formulated above.
Diagrams
T–
s diagram of nitrogen.
[18]
The red curve at the left is the melting curve. The red dome represents
the two-phase region with the low-entropy side the saturated liquid and
the high-entropy side the saturated gas. The black curves give the
T–
s
relation along isobars. The pressures are indicated in bar. The blue
curves are isenthalps (curves of constant enthalpy). The values are
indicated in blue in kJ/kg. The specific points
a,
b, etc., are treated in the main text.
Nowadays the enthalpy values of important substances can be obtained
using commercial software. Practically all relevant material properties
can be obtained either in tabular or in graphical form. There are many
types of diagrams, such as
h–
T diagrams, which give the specific enthalpy as function of temperature for various pressures, and
h–
p diagrams, which give
h as function of
p for various
T. One of the most common diagrams is the temperature–specific entropy diagram (
T–
s-diagram).
It gives the melting curve and saturated liquid and vapor values
together with isobars and isenthalps. These diagrams are powerful tools
in the hands of the thermal engineer.
Some basic applications
The points
a through
h in the figure play a role in the discussion in this section.
- a: T = 300 K, p = 1 bar, s = 6.85 kJ/(kg K), h = 461 kJ/kg;
- b: T = 380 K, p = 2 bar, s = 6.85 kJ/(kg K), h = 530 kJ/kg;
- c: T = 300 K, p = 200 bar, s = 5.16 kJ/(kg K), h = 430 kJ/kg;
- d: T = 270 K, p = 1 bar, s = 6.79 kJ/(kg K), h = 430 kJ/kg;
- e: T = 108 K, p = 13 bar, s = 3.55 kJ/(kg K), h = 100 kJ/kg (saturated liquid at 13 bar);
- f: T = 77.2 K, p = 1 bar, s = 3.75 kJ/(kg K), h = 100 kJ/kg;
- g: T = 77.2 K, p = 1 bar, s = 2.83 kJ/(kg K), h = 28 kJ/kg (saturated liquid at 1 bar);
- h: T = 77.2 K, p = 1 bar, s = 5.41 kJ/(kg K), h = 230 kJ/kg (saturated gas at 1 bar);
Throttling
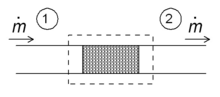
Schematic diagram of a throttling in the steady state. Fluid enters the
system (dotted rectangle) at point 1 and leaves it at point 2. The mass
flow is ṁ.
One of the simple applications of the concept of enthalpy is the so-called throttling process, also known as
Joule-Thomson expansion.
It concerns a steady adiabatic flow of a fluid through a flow
resistance (valve, porous plug, or any other type of flow resistance) as
shown in the figure. This process is very important, since it is at the
heart of domestic refrigerators, where it is responsible for the
temperature drop between ambient temperature and the interior of the
refrigerator. It is also the final stage in many types of liquefiers.
In the first law for open systems (see above) applied to the system,
all terms are zero, except the terms for the enthalpy flow. Hence

Since the mass flow is constant, the specific enthalpies at the two sides of the flow resistance are the same:

that is, the enthalpy per unit mass does not change during the
throttling. The consequences of this relation can be demonstrated using
the
T–
s diagram above. Point
c is at 200 bar and
room temperature (300 K). A Joule–Thomson expansion from 200 bar to
1 bar follows a curve of constant enthalpy of roughly 425 kJ/kg (not
shown in the diagram) lying between the 400 and 450 kJ/kg isenthalps and
ends in point
d, which is at a temperature of about 270 K. Hence
the expansion from 200 bar to 1 bar cools nitrogen from 300 K to 270 K.
In the valve, there is a lot of friction, and a lot of entropy is
produced, but still the final temperature is below the starting value!
Point
e is chosen so that it is on the saturated liquid line with
h = 100 kJ/kg. It corresponds roughly with
p = 13 bar and
T = 108 K. Throttling from this point to a pressure of 1 bar ends in the two-phase region (point
f).
This means that a mixture of gas and liquid leaves the throttling
valve. Since the enthalpy is an extensive parameter, the enthalpy in
f (
hf) is equal to the enthalpy in
g (
hg) multiplied by the liquid fraction in
f (
xf) plus the enthalpy in
h (
hh) multiplied by the gas fraction in
f (1 − xf). So

With numbers:
100 = xf × 28 + (1 − xf) × 230, so
xf = 0.64. This means that the mass fraction of the liquid in the liquid–gas mixture that leaves the throttling valve is 64%.
Compressors

Schematic diagram of a compressor in the steady state. Fluid enters the
system (dotted rectangle) at point 1 and leaves it at point 2. The mass
flow is ṁ. A power P is applied and a heat flow Q̇ is released to the surroundings at ambient temperature Ta.
A power
P is applied e.g. as electrical power. If the
compression is adiabatic, the gas temperature goes up. In the reversible
case it would be at constant entropy, which corresponds with a vertical
line in the
T–
s diagram. For example, compressing nitrogen from 1 bar (point
a) to 2 bar (point
b) would result in a temperature increase from 300 K to 380 K. In order to let the compressed gas exit at ambient temperature
Ta,
heat exchange, e.g. by cooling water, is necessary. In the ideal case
the compression is isothermal. The average heat flow to the surroundings
is
Q̇. Since the system is in the steady state the first law gives

The minimal power needed for the compression is realized if the compression is reversible. In that case the
second law of thermodynamics for open systems gives

Eliminating
Q̇ gives for the minimal power

For example, compressing 1 kg of nitrogen from 1 bar to 200 bar costs at least
(hc − ha) − Ta(sc − sa). With the data, obtained with the
T–
s diagram, we find a value of
(430 − 461) − 300 × (5.16 − 6.85) = 476 kJ/kg.
The relation for the power can be further simplified by writing it as

With
dh = T ds + v dp, this results in the final relation

 years.[13] Over an infinite time, there would be a spontaneous entropy decrease via the Poincaré recurrence theorem[citation needed], thermal fluctuations,[14][15] and Fluctuation theorem.[16][17]
years.[13] Over an infinite time, there would be a spontaneous entropy decrease via the Poincaré recurrence theorem[citation needed], thermal fluctuations,[14][15] and Fluctuation theorem.[16][17]

















































