From Wikipedia, the free encyclopedia
The
second law of thermodynamics states that the total
entropy of an
isolated system
can never decrease over time. The total entropy of a system and its
surroundings can remain constant in ideal cases where the system is in
thermodynamic equilibrium, or is undergoing a (fictive)
reversible process. In all processes that occur, including
spontaneous processes, the total entropy of the system and its surroundings increases and the process is
irreversible in the thermodynamic sense. The increase in entropy accounts for the irreversibility of natural processes, and the
asymmetry between future and past.
Historically, the second law was an
empirical finding that was accepted as an axiom of
thermodynamic theory.
Statistical mechanics, classical or
quantum, explains the microscopic origin of the law.
The second law has been expressed in many ways. Its first formulation is credited to the French scientist
Sadi Carnot, who in 1824 showed that there is an upper limit to the efficiency of conversion of heat to work, in a heat engine.
Introduction

Heat flow from hot water to cold water.
The
first law of thermodynamics provides the basic definition of
internal energy, associated with all
thermodynamic systems, and states the rule of
conservation of energy. The second law is concerned with the direction of natural processes.
It asserts that a natural process runs only in one sense, and is not
reversible. For example, heat always flows spontaneously from hotter to
colder bodies, and never the reverse, unless external work is performed
on the system. The explanation of the phenomena was given in terms of
entropy. Total entropy (
S)
can never decrease over time for an isolated system because the entropy
of an isolated system spontaneously evolves toward thermodynamic
equilibrium: the entropy should stay the same or increase.
In a fictive reversible process, an infinitesimal increment in the entropy (
dS) of a system is defined to result from an infinitesimal transfer of heat (
δQ) to a
closed system (which allows the entry or exit of energy – but not
mass transfer) divided by the common temperature (
T) of the system in equilibrium and the surroundings which supply the heat:

Different notations are used for infinitesimal amounts of heat (
δ) and infinitesimal amounts of entropy (
d) because entropy is a
function of state,
while heat, like work, is not. For an actually possible infinitesimal
process without exchange of mass with the surroundings, the second law
requires that the increment in system entropy fulfills the
inequality

This is because a general process for this case may include work
being done on the system by its surroundings, which can have frictional
or viscous effects inside the system, because a chemical reaction may be
in progress, or because heat transfer actually occurs only
irreversibly, driven by a finite difference between the system
temperature (T) and the temperature of the surroundings (Tsurr). Note that the equality still applies for pure heat flow,

which is the basis of the accurate determination of the absolute
entropy of pure substances from measured heat capacity curves and
entropy changes at phase transitions, i.e. by calorimetry. Introducing a set of internal variables

to describe the deviation of a thermodynamic system in physical equilibrium (with the required well-defined uniform pressure
P and temperature
T) from the chemical equilibrium state, one can record the equality

The second term represents work of internal variables that can be
perturbed by external influences, but the system cannot perform any
positive work via internal variables. This statement introduces the
impossibility of the reversion of evolution of the thermodynamic system
in time and can be considered as a formulation of the second principle of thermodynamics – the formulation, which is, of course, equivalent to the formulation of the principle in terms of entropy.
The
zeroth law of thermodynamics
in its usual short statement allows recognition that two bodies in a
relation of thermal equilibrium have the same temperature, especially
that a test body has the same temperature as a reference thermometric
body.
For a body in thermal equilibrium with another, there are indefinitely
many empirical temperature scales, in general respectively depending on
the properties of a particular reference thermometric body. The second
law allows a distinguished temperature scale, which defines an absolute,
thermodynamic temperature, independent of the properties of any particular reference thermometric body.
Various statements of the law
The second law of thermodynamics may be expressed in many specific ways, the most prominent classical statements being the statement by
Rudolf Clausius (1854), the statement by
Lord Kelvin (1851), and the statement in axiomatic thermodynamics by
Constantin Carathéodory
(1909). These statements cast the law in general physical terms citing
the impossibility of certain processes. The Clausius and the Kelvin
statements have been shown to be equivalent.
Carnot's principle
The historical origin of the second law of thermodynamics was in Carnot's principle. It refers to a cycle of a
Carnot heat engine,
fictively operated in the limiting mode of extreme slowness known as
quasi-static, so that the heat and work transfers are between subsystems
that are always in their own internal states of thermodynamic
equilibrium. The Carnot engine is an idealized device of special
interest to engineers who are concerned with the efficiency of heat
engines. Carnot's principle was recognized by Carnot at a time when the
caloric theory of heat was seriously considered, before the recognition of the
first law of thermodynamics,
and before the mathematical expression of the concept of entropy.
Interpreted in the light of the first law, it is physically equivalent
to the second law of thermodynamics, and remains valid today. It states
The efficiency of a quasi-static or reversible Carnot
cycle depends only on the temperatures of the two heat reservoirs, and
is the same, whatever the working substance. A Carnot engine operated in
this way is the most efficient possible heat engine using those two
temperatures.
Clausius statement
The German scientist
Rudolf Clausius laid the foundation for the second law of thermodynamics in 1850 by examining the relation between heat transfer and work. His formulation of the second law, which was published in German in 1854, is known as the
Clausius statement:
Heat can never pass from a colder to a warmer body without some other change, connected therewith, occurring at the same time.
The statement by Clausius uses the concept of 'passage of heat'. As
is usual in thermodynamic discussions, this means 'net transfer of
energy as heat', and does not refer to contributory transfers one way
and the other.
Heat cannot spontaneously flow from cold regions to hot regions
without external work being performed on the system, which is evident
from ordinary experience of refrigeration, for example. In a
refrigerator, heat flows from cold to hot, but only when forced by an
external agent, the refrigeration system.
Kelvin statement
It is impossible, by means of inanimate material agency,
to derive mechanical effect from any portion of matter by cooling it
below the temperature of the coldest of the surrounding objects.
Equivalence of the Clausius and the Kelvin statements
Derive Kelvin Statement from Clausius Statement
Suppose there is an engine violating the Kelvin statement: i.e., one
that drains heat and converts it completely into work in a cyclic
fashion without any other result. Now pair it with a reversed
Carnot engine
as shown by the figure. The net and sole effect of this newly created
engine consisting of the two engines mentioned is transferring heat

from the cooler reservoir to the hotter one, which violates the Clausius statement(This is a consequence of the
first law of thermodynamics, as for the total system's energy to remain the same,

, so therefore

). Thus a violation of the Kelvin statement implies a violation of the
Clausius statement, i.e. the Clausius statement implies the Kelvin
statement. We can prove in a similar manner that the Kelvin statement
implies the Clausius statement, and hence the two are equivalent.
Planck's proposition
Planck
offered the following proposition as derived directly from experience.
This is sometimes regarded as his statement of the second law, but he
regarded it as a starting point for the derivation of the second law.
It is impossible to construct an engine which will work
in a complete cycle, and produce no effect except the raising of a
weight and cooling of a heat reservoir.
Relation between Kelvin's statement and Planck's proposition
The Kelvin–Planck statement (or the heat engine statement) of the second law of thermodynamics states that
It is impossible to devise a cyclically operating device, the sole effect of which is to absorb energy in the form of heat from a single thermal reservoir and to deliver an equivalent amount of work.
Planck's statement
Planck stated the second law as follows.
Every process occurring in nature proceeds in the sense
in which the sum of the entropies of all bodies taking part in the
process is increased. In the limit, i.e. for reversible processes, the
sum of the entropies remains unchanged.
Rather like Planck's statement is that of Uhlenbeck and Ford for irreversible phenomena.
... in an irreversible or spontaneous change from one
equilibrium state to another (as for example the equalization of
temperature of two bodies A and B, when brought in contact) the entropy
always increases.
Principle of Carathéodory
Constantin Carathéodory
formulated thermodynamics on a purely mathematical axiomatic
foundation. His statement of the second law is known as the Principle of
Carathéodory, which may be formulated as follows:
In every neighborhood of any state S of an adiabatically enclosed system there are states inaccessible from S.
With this formulation, he described the concept of
adiabatic accessibility for the first time and provided the foundation for a new subfield of classical thermodynamics, often called
geometrical thermodynamics. It follows from Carathéodory's principle that quantity of energy quasi-statically transferred as heat is a holonomic
process function, in other words,

.
Though it is almost customary in textbooks to say that
Carathéodory's principle expresses the second law and to treat it as
equivalent to the Clausius or to the Kelvin-Planck statements, such is
not the case. To get all the content of the second law, Carathéodory's
principle needs to be supplemented by Planck's principle, that isochoric
work always increases the internal energy of a closed system that was
initially in its own internal thermodynamic equilibrium.
Planck's principle
In 1926,
Max Planck wrote an important paper on the basics of thermodynamics. He indicated the principle
The internal energy of a closed system is increased by
an adiabatic process, throughout the duration of which, the volume of
the system remains constant.
This formulation does not mention heat and does not mention
temperature, nor even entropy, and does not necessarily implicitly rely
on those concepts, but it implies the content of the second law. A
closely related statement is that "Frictional pressure never does
positive work." Using a now-obsolete form of words, Planck himself wrote: "The production of heat by friction is irreversible."
Not mentioning entropy, this principle of Planck is stated in
physical terms. It is very closely related to the Kelvin statement given
just above. It is relevant that for a system at constant volume and
mole numbers,
the entropy is a monotonic function of the internal energy.
Nevertheless, this principle of Planck is not actually Planck's
preferred statement of the second law, which is quoted above, in a
previous sub-section of the present section of this present article, and
relies on the concept of entropy.
A statement that in a sense is complementary to Planck's
principle is made by Borgnakke and Sonntag. They do not offer it as a
full statement of the second law:
... there is only one way in which the entropy of a
[closed] system can be decreased, and that is to transfer heat from the
system.
Differing from Planck's just foregoing principle, this one is
explicitly in terms of entropy change. Removal of matter from a system
can also decrease its entropy.
Statement for a system that has a known expression of its internal energy as a function of its extensive state variables
The second law has been shown to be equivalent to the
internal energy U being a weakly
convex function, when written as a function of extensive properties (mass, volume, entropy, ...).
Corollaries
Perpetual motion of the second kind
Before the establishment of the second law, many people who were
interested in inventing a perpetual motion machine had tried to
circumvent the restrictions of
first law of thermodynamics
by extracting the massive internal energy of the environment as the
power of the machine. Such a machine is called a "perpetual motion
machine of the second kind". The second law declared the impossibility
of such machines.
Carnot theorem
Carnot's theorem
(1824) is a principle that limits the maximum efficiency for any
possible engine. The efficiency solely depends on the temperature
difference between the hot and cold thermal reservoirs. Carnot's theorem
states:
- All irreversible heat engines between two heat reservoirs are less efficient than a Carnot engine operating between the same reservoirs.
- All reversible heat engines between two heat reservoirs are equally
efficient with a Carnot engine operating between the same reservoirs.
In his ideal model, the heat of caloric converted into work could be
reinstated by reversing the motion of the cycle, a concept subsequently
known as
thermodynamic reversibility.
Carnot, however, further postulated that some caloric is lost, not
being converted to mechanical work. Hence, no real heat engine could
realise the
Carnot cycle's reversibility and was condemned to be less efficient.
Though formulated in terms of caloric, rather than
entropy, this was an early insight into the second law.
Clausius inequality

The equality holds in the reversible case and the strict inequality holds in the irreversible case. The reversible case is used to introduce the state function
entropy. This is because in cyclic processes the variation of a state function is zero from state functionality.
Thermodynamic temperature
For an arbitrary heat engine, the efficiency is:
 ,
,
where Wn is for the net work done per cycle. Thus the efficiency depends only on qC/qH.
Carnot's theorem states that all reversible engines operating between the same heat reservoirs are equally efficient.
Thus, any reversible heat engine operating between temperatures
T1 and
T2 must have the same efficiency, that is to say, the efficiency is the function of temperatures only:

In addition, a reversible heat engine operating between temperatures T1 and T3 must have the same efficiency as one consisting of two cycles, one between T1 and another (intermediate) temperature T2, and the second between T2 andT3. This can only be the case if

Now consider the case where

is a fixed reference temperature: the temperature of the
triple point of water. Then for any
T2 and
T3,

Therefore, if thermodynamic temperature is defined by
 ,
,
then the function f, viewed as a function of thermodynamic temperature, is simply

and the reference temperature
T1 will have the
value 273.16. (Any reference temperature and any positive numerical
value could be used – the choice here corresponds to the
Kelvin scale.)
Entropy
 .
.
That means the line integral

is path independent for reversible processes. So we can define a state function S called entropy, which for a reversible process or for pure heat transfer satisfies
 .
.
With this we can only obtain the difference of entropy by integrating
the above formula. To obtain the absolute value, we need the
third law of thermodynamics, which states that
S = 0 at
absolute zero for perfect crystals.
For any irreversible process, since entropy is a state function,
we can always connect the initial and terminal states with an imaginary
reversible process and integrating on that path to calculate the
difference in entropy.
Now reverse the reversible process and combine it with the said irreversible process. Applying the
Clausius inequality on this loop,
 .
.
Thus,
 ,
,
where the equality holds if the transformation is reversible.
Notice that if the process is an
adiabatic process, then

, so

.
Energy, available useful work
An important and revealing idealized special case is to consider
applying the Second Law to the scenario of an isolated system (called
the total system or universe), made up of two parts: a sub-system of
interest, and the sub-system's surroundings. These surroundings are
imagined to be so large that they can be considered as an unlimited heat reservoir at temperature TR and pressure PR – so that no matter how much heat is transferred to (or from) the sub-system, the temperature of the surroundings will remain TR; and no matter how much the volume of the sub-system expands (or contracts), the pressure of the surroundings will remain PR.
Whatever changes to dS and dSR occur in the entropies of the sub-system and the surroundings individually, according to the Second Law the entropy Stot of the isolated total system must not decrease:
 .
.
According to the
first law of thermodynamics, the change
dU in the internal energy of the sub-system is the sum of the heat
δq added to the sub-system,
less any work
δw done
by the sub-system,
plus any net chemical energy entering the sub-system
d ∑μiRNi, so that:
 ,
,
where μ
iR are the
chemical potentials of chemical species in the external surroundings. Now the heat leaving the reservoir and entering the sub-system is
 ,
,
where we have first used the definition of entropy in classical
thermodynamics (alternatively, in statistical thermodynamics, the
relation between entropy change, temperature and absorbed heat can be
derived); and then the Second Law inequality from above.
It therefore follows that any net work δw done by the sub-system must obey
 .
.
It is useful to separate the work δw done by the subsystem into the useful work δwu that can be done by the sub-system, over and beyond the work pR dV
done merely by the sub-system expanding against the surrounding
external pressure, giving the following relation for the useful work
(exergy) that can be done:
 .
.
It is convenient to define the right-hand-side as the exact derivative of a thermodynamic potential, called the
availability or
exergy E of the subsystem,
 .
.
The Second Law therefore implies that for any process which can be
considered as divided simply into a subsystem, and an unlimited
temperature and pressure reservoir with which it is in contact,
 ,
,
i.e. the change in the subsystem's exergy plus the useful work done by the subsystem (or, the change in the subsystem's exergy less any work, additional to that done by the pressure reservoir, done on the system) must be less than or equal to zero.
In sum, if a proper infinite-reservoir-like reference state is chosen as the system surroundings in the real world, then the Second Law predicts a decrease in E for an irreversible process and no change for a reversible process.
 Is equivalent to
Is equivalent to  .
.
This expression together with the associated reference state permits a
design engineer working at the macroscopic scale (above the
thermodynamic limit) to utilize the Second Law without directly measuring or considering entropy change in a total isolated system. Those changes have already been considered by the assumption that the
system under consideration can reach equilibrium with the reference
state without altering the reference state. An efficiency for a process
or collection of processes that compares it to the reversible ideal may
also be found.
The second law in chemical thermodynamics
 ,
,
or d
G < 0. For a similar process at constant temperature and volume, the change in
Helmholtz free energy must be negative,

.
Thus, a negative value of the change in free energy (G or A) is a
necessary condition for a process to be spontaneous. This is the most
useful form of the second law of thermodynamics in chemistry, where
free-energy changes can be calculated from tabulated enthalpies of
formation and standard molar entropies of reactants and products. The chemical equilibrium condition at constant
T and
p without electrical work is d
G = 0.
History
Nicolas Léonard Sadi Carnot in the traditional uniform of a student of the École Polytechnique.
The first theory of the conversion of heat into mechanical work is due to
Nicolas Léonard Sadi Carnot
in 1824. He was the first to realize correctly that the efficiency of
this conversion depends on the difference of temperature between an
engine and its environment.
Recognizing the significance of
James Prescott Joule's work on the conservation of energy,
Rudolf Clausius was the first to formulate the second law during 1850, in this form: heat does not flow
spontaneously from cold to hot bodies. While common knowledge now, this was contrary to the
caloric theory
of heat popular at the time, which considered heat as a fluid. From
there he was able to infer the principle of Sadi Carnot and the
definition of entropy (1865).
Established during the 19th century, the
Kelvin-Planck statement of the Second Law says, "It is impossible for any device that operates on a
cycle to receive heat from a single
reservoir and produce a net amount of work." This was shown to be equivalent to the statement of Clausius.
The
ergodic hypothesis is also important for the
Boltzmann
approach. It says that, over long periods of time, the time spent in
some region of the phase space of microstates with the same energy is
proportional to the volume of this region, i.e. that all accessible
microstates are equally probable over a long period of time.
Equivalently, it says that time average and average over the statistical
ensemble are the same.
There is a traditional doctrine, starting with Clausius, that
entropy can be understood in terms of molecular 'disorder' within a
macroscopic system. This doctrine is obsolescent.
Account given by Clausius
 ,
,
where Q is heat, T is temperature and N is the
"equivalence-value" of all uncompensated transformations involved in a
cyclical process. Later, in 1865, Clausius would come to define
"equivalence-value" as entropy. On the heels of this definition, that
same year, the most famous version of the second law was read in a
presentation at the Philosophical Society of Zurich on April 24, in
which, in the end of his presentation, Clausius concludes:
The entropy of the universe tends to a maximum.
This statement is the best-known phrasing of the second law. Because of the looseness of its language, e.g.
universe,
as well as lack of specific conditions, e.g. open, closed, or isolated,
many people take this simple statement to mean that the second law of
thermodynamics applies virtually to every subject imaginable. This is
not true; this statement is only a simplified version of a more extended
and precise description.
In terms of time variation, the mathematical statement of the second law for an
isolated system undergoing an arbitrary transformation is:
 ,
,
where
- S is the entropy of the system and
- t is time.
The equality sign applies after equilibration. An alternative way of formulating of the second law for isolated systems is:
 with
with  ,
,
with

the sum of the rate of
entropy production by all processes inside the system. The advantage of this formulation is that it shows the effect of the
entropy production.
The rate of entropy production is a very important concept since it
determines (limits) the efficiency of thermal machines. Multiplied with
ambient temperature

it gives the so-called dissipated energy

.
The expression of the second law for closed systems (so, allowing
heat exchange and moving boundaries, but not exchange of matter) is:
 with
with  .
.
Here
 is the heat flow into the system
is the heat flow into the system is the temperature at the point where the heat enters the system.
is the temperature at the point where the heat enters the system.
The equality sign holds in the case that only reversible processes
take place inside the system. If irreversible processes take place
(which is the case in real systems in operation) the >-sign holds.
If heat is supplied to the system at several places we have to take the
algebraic sum of the corresponding terms.
For open systems (also allowing exchange of matter):
 with
with  .
.
Here

is the flow of entropy into the system associated with the flow of
matter entering the system. It should not be confused with the time
derivative of the entropy. If matter is supplied at several places we
have to take the algebraic sum of these contributions.
Statistical mechanics
Statistical mechanics
gives an explanation for the second law by postulating that a material
is composed of atoms and molecules which are in constant motion. A
particular set of positions and velocities for each particle in the
system is called a
microstate
of the system and because of the constant motion, the system is
constantly changing its microstate. Statistical mechanics postulates
that, in equilibrium, each microstate that the system might be in is
equally likely to occur, and when this assumption is made, it leads
directly to the conclusion that the second law must hold in a
statistical sense. That is, the second law will hold on average, with a
statistical variation on the order of 1/
√N where
N
is the number of particles in the system. For everyday (macroscopic)
situations, the probability that the second law will be violated is
practically zero. However, for systems with a small number of particles,
thermodynamic parameters, including the entropy, may show significant
statistical deviations from that predicted by the second law. Classical
thermodynamic theory does not deal with these statistical variations.
Derivation from statistical mechanics
Due to
Loschmidt's paradox, derivations of the Second Law have to make an assumption regarding the past, namely that the system is
uncorrelated at some time in the past; this allows for simple probabilistic treatment. This assumption is usually thought as a
boundary condition,
and thus the second Law is ultimately a consequence of the initial
conditions somewhere in the past, probably at the beginning of the
universe (the
Big Bang), though
other scenarios have also been suggested.
Given these assumptions, in statistical mechanics, the Second Law is not a postulate, rather it is a consequence of the
fundamental postulate,
also known as the equal prior probability postulate, so long as one is
clear that simple probability arguments are applied only to the future,
while for the past there are auxiliary sources of information which tell
us that it was low entropy.
The first part of the second law, which states that the entropy of a
thermally isolated system can only increase, is a trivial consequence of
the equal prior probability postulate, if we restrict the notion of the
entropy to systems in thermal equilibrium. The entropy of an isolated
system in thermal equilibrium containing an amount of energy of

is:
![S = k_{\mathrm B} \ln\left[\Omega\left(E\right)\right]\,](https://wikimedia.org/api/rest_v1/media/math/render/svg/bb924dffe4f5c2c580cd40461cf5bfc5159ac881) ,
,
where

is the number of quantum states in a small interval between

and

. Here

is a macroscopically small energy interval that is kept fixed. Strictly
speaking this means that the entropy depends on the choice of

.
However, in the thermodynamic limit (i.e. in the limit of infinitely
large system size), the specific entropy (entropy per unit volume or per
unit mass) does not depend on

.
Suppose we have an isolated system whose macroscopic state is
specified by a number of variables. These macroscopic variables can,
e.g., refer to the total volume, the positions of pistons in the system,
etc. Then

will depend on the values of these variables. If a variable is not
fixed, (e.g. we do not clamp a piston in a certain position), then
because all the accessible states are equally likely in equilibrium, the
free variable in equilibrium will be such that

is maximized as that is the most probable situation in equilibrium.
If the variable was initially fixed to some value then upon
release and when the new equilibrium has been reached, the fact the
variable will adjust itself so that

is maximized, implies that the entropy will have increased or it will
have stayed the same (if the value at which the variable was fixed
happened to be the equilibrium value).
Suppose we start from an equilibrium situation and we suddenly remove a
constraint on a variable. Then right after we do this, there are a
number

of accessible microstates, but equilibrium has not yet been reached, so
the actual probabilities of the system being in some accessible state
are not yet equal to the prior probability of

.
We have already seen that in the final equilibrium state, the entropy
will have increased or have stayed the same relative to the previous
equilibrium state. Boltzmann's
H-theorem, however, proves that the quantity
H increases monotonically as a function of time during the intermediate out of equilibrium state.
Derivation of the entropy change for reversible processes
The second part of the Second Law states that the entropy change of a system undergoing a reversible process is given by:
 ,
,
where the temperature is defined as:
![\frac{1}{k_{\mathrm B} T}\equiv\beta\equiv\frac{d\ln\left[\Omega\left(E\right)\right]}{dE}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e720432da60639a2f9cdcefb4ac56845da4f36b0) .
.
Suppose that the system has
some external parameter, x, that can be changed. In general, the energy
eigenstates of the system will depend on x. According to the
adiabatic theorem
of quantum mechanics, in the limit of an infinitely slow change of the
system's Hamiltonian, the system will stay in the same energy eigenstate
and thus change its energy according to the change in energy of the
energy eigenstate it is in.
The generalized force, X, corresponding to the external variable x is defined such that

is the work performed by the system if x is increased by an amount dx.
E.g., if x is the volume, then X is the pressure. The generalized force
for a system known to be in energy eigenstate

is given by:
 .
.
Since the system can be in any energy eigenstate within an interval of

, we define the generalized force for the system as the expectation value of the above expression:
 .
.
To evaluate the average, we partition the

energy eigenstates by counting how many of them have a value for

within a range between

and

. Calling this number

, we have:
 .
.
The average defining the generalized force can now be written:
 .
.
We can relate this to the derivative of the entropy with respect to x
at constant energy E as follows. Suppose we change x to x + dx. Then

will change because the energy eigenstates depend on x, causing energy eigenstates to move into or out of the range between

and

. Let's focus again on the energy eigenstates for which

lies within the range between

and

.
Since these energy eigenstates increase in energy by Y dx, all such
energy eigenstates that are in the interval ranging from E – Y dx to E
move from below E to above E. There are

such energy eigenstates. If

, all these energy eigenstates will move into the range between

and

and contribute to an increase in

. The number of energy eigenstates that move from below

to above

is given by

. The difference

is thus the net contribution to the increase in

. Note that if Y dx is larger than

there will be the energy eigenstates that move from below E to above

. They are counted in both

and

, therefore the above expression is also valid in that case.
Expressing the above expression as a derivative with respect to E and summing over Y yields the expression:
 .
.
The logarithmic derivative of

with respect to x is thus given by:
 .
.
The first term is intensive, i.e. it does not scale with system size.
In contrast, the last term scales as the inverse system size and will
thus vanishes in the thermodynamic limit. We have thus found that:
 .
.
Combining this with

gives:
 .
.
Derivation for systems described by the canonical ensemble
If
a system is in thermal contact with a heat bath at some temperature T
then, in equilibrium, the probability distribution over the energy
eigenvalues are given by the
canonical ensemble:
 .
.
Here Z is a factor that normalizes the sum of all the probabilities to 1, this function is known as the
partition function.
We now consider an infinitesimal reversible change in the temperature
and in the external parameters on which the energy levels depend. It
follows from the general formula for the entropy:
 ,
,
that
 .
.
Inserting the formula for

for the canonical ensemble in here gives:
 .
.
Living organisms
There
are two principal ways of formulating thermodynamics, (a) through
passages from one state of thermodynamic equilibrium to another, and (b)
through cyclic processes, by which the system is left unchanged, while
the total entropy of the surroundings is increased. These two ways help
to understand the processes of life. This topic is mostly beyond the
scope of this present article, but has been considered by several
authors, such as
Erwin Schrödinger,
Léon Brillouin and
Isaac Asimov. It is also the topic of current research.
To a fair approximation, living organisms may be considered as
examples of (b). Approximately, an animal's physical state cycles by the
day, leaving the animal nearly unchanged. Animals take in food, water,
and oxygen, and, as a result of
metabolism, give out breakdown products and heat. Plants
take in radiative energy
from the sun, which may be regarded as heat, and carbon dioxide and
water. They give out oxygen. In this way they grow. Eventually they die,
and their remains rot away, turning mostly back into carbon dioxide and
water. This can be regarded as a cyclic process. Overall, the sunlight
is from a high temperature source, the sun, and its energy is passed to a
lower temperature sink, i.e. radiated into space. This is an increase
of entropy of the surroundings of the plant. Thus animals and plants
obey the second law of thermodynamics, considered in terms of cyclic
processes. Simple concepts of efficiency of heat engines are hardly
applicable to this problem because they assume closed systems.
From the thermodynamic viewpoint that considers (a), passages
from one equilibrium state to another, only a roughly approximate
picture appears, because living organisms are never in states of
thermodynamic equilibrium. Living organisms must often be considered as
open systems, because they take in nutrients and give out waste
products. Thermodynamics of open systems is currently often considered
in terms of passages from one state of thermodynamic equilibrium to
another, or in terms of flows in the approximation of local
thermodynamic equilibrium. The problem for living organisms may be
further simplified by the approximation of assuming a steady state with
unchanging flows. General principles of entropy production for such
approximations are subject to
unsettled current debate or research. Nevertheless, ideas derived from this viewpoint on the second law of thermodynamics are enlightening about living creatures.
Gravitational systems
In systems that do not require for their descriptions the general theory of relativity, bodies always have positive
heat capacity,
meaning that the temperature rises with energy. Therefore, when energy
flows from a high-temperature object to a low-temperature object, the
source temperature is decreased while the sink temperature is increased;
hence temperature differences tend to diminish over time. This is not
always the case for systems in which the gravitational force is
important and the general theory of relativity is required. Such systems
can spontaneously change towards uneven spread of mass and energy. This
applies to the universe in large scale, and consequently it may be
difficult or impossible to apply the second law to it.
Beyond this, the thermodynamics of systems described by the general
theory of relativity is beyond the scope of the present article.
Non-equilibrium states
The theory of classical or
equilibrium thermodynamics
is idealized. A main postulate or assumption, often not even explicitly
stated, is the existence of systems in their own internal states of
thermodynamic equilibrium. In general, a region of space containing a
physical system at a given time, that may be found in nature, is not in
thermodynamic equilibrium, read in the most stringent terms. In looser
terms, nothing in the entire universe is or has ever been truly in exact
thermodynamic equilibrium.
For purposes of physical analysis, it is often enough convenient to make an assumption of
thermodynamic equilibrium.
Such an assumption may rely on trial and error for its justification.
If the assumption is justified, it can often be very valuable and useful
because it makes available the theory of thermodynamics. Elements of
the equilibrium assumption are that a system is observed to be
unchanging over an indefinitely long time, and that there are so many
particles in a system, that its particulate nature can be entirely
ignored. Under such an equilibrium assumption, in general, there are no
macroscopically detectable
fluctuations. There is an exception, the case of
critical states, which exhibit to the naked eye the phenomenon of
critical opalescence. For laboratory studies of critical states, exceptionally long observation times are needed.
In all cases, the assumption of
thermodynamic equilibrium, once made, implies as a consequence that no putative candidate "fluctuation" alters the entropy of the system.
It can easily happen that a physical system exhibits internal
macroscopic changes that are fast enough to invalidate the assumption of
the constancy of the entropy. Or that a physical system has so few
particles that the particulate nature is manifest in observable
fluctuations. Then the assumption of thermodynamic equilibrium is to be
abandoned. There is no unqualified general definition of entropy for
non-equilibrium states.
There are intermediate cases, in which the assumption of local
thermodynamic equilibrium is a very good approximation,
but strictly speaking it is still an approximation, not theoretically
ideal. For non-equilibrium situations in general, it may be useful to
consider statistical mechanical definitions of other quantities that may
be conveniently called 'entropy', but they should not be confused or
conflated with thermodynamic entropy properly defined for the second
law. These other quantities indeed belong to statistical mechanics, not
to thermodynamics, the primary realm of the second law.
Even though the applicability of the second law of thermodynamics
is limited for non-equilibrium systems, the laws governing such systems
are still being discussed. One of the guiding principles for systems
which are far from equilibrium is the maximum entropy production
principle.
It states that a system away from equilibrium evolves in such a way as
to maximize entropy production, given present constraints.
The physics of macroscopically observable fluctuations is beyond the scope of this article.
Arrow of time
The second law of thermodynamics is a physical law that is not
symmetric to reversal of the time direction. This does not conflict with
notions that have been observed of the fundamental laws of physics,
namely
CPT symmetry, since the second law applies statistically, it is hypothesized, on time-asymmetric
boundary conditions.
The second law has been proposed to supply a partial explanation
of the difference between moving forward and backwards in time, such as
why the cause precedes the effect.
Irreversibility
Irreversibility in
thermodynamic processes
is a consequence of the asymmetric character of thermodynamic
operations, and not of any internally irreversible microscopic
properties of the bodies. Thermodynamic operations are macroscopic
external interventions imposed on the participating bodies, not derived
from their internal properties. There are reputed "paradoxes" that arise
from failure to recognize this.
Loschmidt's paradox
Loschmidt's paradox,
also known as the reversibility paradox, is the objection that it
should not be possible to deduce an irreversible process from the
time-symmetric dynamics that describe the microscopic evolution of a
macroscopic system.
In the opinion of
Schrödinger,
"It is now quite obvious in what manner you have to reformulate the law
of entropy – or for that matter, all other irreversible statements – so
that they be capable of being derived from reversible models. You must
not speak of one isolated system but at least of two, which you may for
the moment consider isolated from the rest of the world, but not always
from each other."
The two systems are isolated from each other by the wall, until it is
removed by the thermodynamic operation, as envisaged by the law. The
thermodynamic operation is externally imposed, not subject to the
reversible microscopic dynamical laws that govern the constituents of
the systems. It is the cause of the irreversibility. The statement of
the law in this present article complies with Schrödinger's advice. The
cause–effect relation is logically prior to the second law, not derived
from it.
Poincaré recurrence theorem
The
Poincaré recurrence theorem
considers a theoretical microscopic description of an isolated physical
system. This may be considered as a model of a thermodynamic system
after a thermodynamic operation has removed an internal wall. The system
will, after a sufficiently long time, return to a microscopically
defined state very close to the initial one. The Poincaré recurrence
time is the length of time elapsed until the return. It is exceedingly
long, likely longer than the life of the universe, and depends
sensitively on the geometry of the wall that was removed by the
thermodynamic operation. The recurrence theorem may be perceived as
apparently contradicting the second law of thermodynamics. More
obviously, however, it is simply a microscopic model of thermodynamic
equilibrium in an isolated system formed by removal of a wall between
two systems. For a typical thermodynamical system, the recurrence time
is so large (many many times longer than the lifetime of the universe)
that, for all practical purposes, one cannot observe the recurrence. One
might wish, nevertheless, to imagine that one could wait for the
Poincaré recurrence, and then re-insert the wall that was removed by the
thermodynamic operation. It is then evident that the appearance of
irreversibility is due to the utter unpredictability of the Poincaré
recurrence given only that the initial state was one of thermodynamic
equilibrium, as is the case in macroscopic thermodynamics. Even if one
could wait for it, one has no practical possibility of picking the right
instant at which to re-insert the wall. The Poincaré recurrence theorem
provides a solution to Loschmidt's paradox. If an isolated
thermodynamic system could be monitored over increasingly many multiples
of the average Poincaré recurrence time, the thermodynamic behavior of
the system would become invariant under time reversal.
Maxwell's demon
James Clerk Maxwell imagined one container divided into two parts,
A and
B. Both parts are filled with the same
gas at equal temperatures and placed next to each other, separated by a wall. Observing the
molecules on both sides, an imaginary
demon guards a microscopic trapdoor in the wall. When a faster-than-average molecule from
A flies towards the trapdoor, the demon opens it, and the molecule will fly from
A to
B. The average
speed of the molecules in
B will have increased while in
A they will have slowed down on average. Since average molecular speed corresponds to temperature, the temperature decreases in
A and increases in
B, contrary to the second law of thermodynamics.
One response to this question was suggested in 1929 by
Leó Szilárd and later by
Léon Brillouin.
Szilárd pointed out that a real-life Maxwell's demon would need to have
some means of measuring molecular speed, and that the act of acquiring
information would require an expenditure of energy.
Maxwell's 'demon' repeatedly alters the permeability of the wall between
A and
B. It is therefore performing
thermodynamic operations on a microscopic scale, not just observing ordinary spontaneous or natural macroscopic thermodynamic processes.
Quotations
The law that entropy always increases holds, I think, the supreme position among the laws of Nature. If someone points out to you that your pet theory of the universe is in disagreement with Maxwell's equations
– then so much the worse for Maxwell's equations. If it is found to be
contradicted by observation – well, these experimentalists do bungle
things sometimes. But if your theory is found to be against the second
law of thermodynamics I can give you no hope; there is nothing for it
but to collapse in deepest humiliation.
There have been nearly as many formulations of the second law as there have been discussions of it.
Clausius is the author of the
sibyllic utterance, "The energy of the universe is constant; the entropy
of the universe tends to a maximum." The objectives of continuum
thermomechanics stop far short of explaining the "universe", but within
that theory we may easily derive an explicit statement in some ways
reminiscent of Clausius, but referring only to a modest object: an
isolated body of finite size.
— Truesdell, C., Muncaster, R.G. (1980). Fundamentals of Maxwell's Kinetic Theory of a Simple Monatomic Gas, Treated as a Branch of Rational Mechanics, Academic Press, New York, ISBN 0-12-701350-4, p. 17.
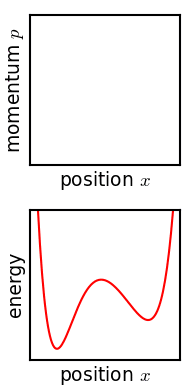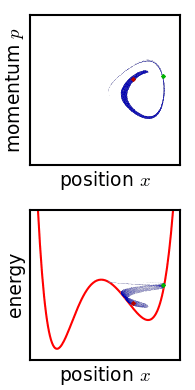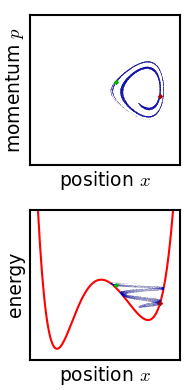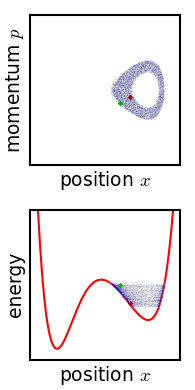








 .
.




 ,
, ,
, .
.
 ,
, ,
, ,
,









 ,
,


 ,
,
 .
.
 .
. .
. ,
,

 .
. ,
, ,
, .
. .
. .
. ,
,
 .
. ,
,


 ,
, ,
,
 ,
,








![S = k_{\mathrm B} \ln\left[\Omega\left(E\right)\right]\,](https://wikimedia.org/api/rest_v1/media/math/render/svg/bb924dffe4f5c2c580cd40461cf5bfc5159ac881) ,
,




 ,
,![\frac{1}{k_{\mathrm B} T}\equiv\beta\equiv\frac{d\ln\left[\Omega\left(E\right)\right]}{dE}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e720432da60639a2f9cdcefb4ac56845da4f36b0) .
.

 .
. .
.



 .
. .
.




 .
. .
.
 .
. ,
, .
.

