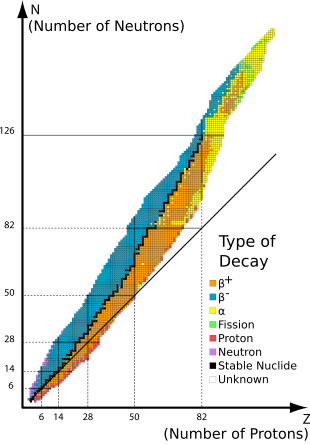From Wikipedia, the free encyclopedia
The environmental isotopes are a subset of the isotopes, both stable and radioactive, which are the object of isotope geochemistry. They are primarily used as tracers to see how things move around within the ocean-atmosphere system, within terrestrial biomes, within the Earth's surface, and between these broad domains.
Isotope Geochemistry
Chemical
elements are defined by their number of protons, but the mass of the
atom is determined by the number of protons and neutrons in the nucleus.
Isotopes are atoms that are of a specific element, but have different
numbers of neutrons and thus different masses. In a specific object, you
can have a ratio between two isotopes of an element. This ratio varies
slightly in the world, so in order to study isotopic ratio changes
across the world, changes in isotope ratios are defined as deviations
from a standard, multiplied by 1000. This unit is a "per mil". As a
convention, the ratio is of the heavier isotope to the lower isotope.
 ‰
‰
These variations in isotopes can occur through many types of
fractionation. They are generally classified as mass independent
fractionation and mass dependent fractionation. An example of a mass
independent process is the fractionation of oxygen atoms in ozone. This
is due to the kinetic isotope effect (KIE) and is caused by different isotope molecules reacting at different speeds.
An example of a mass dependent process is the fractionation of water as
it transitions from the liquid to gas phase. Water molecules with
heavier isotopes (18O and 2H) tend to stay in the liquid phase as water molecules with lighter isotopes (16O and 1H) preferentially move to the gas phase.
Of the different isotopes that exist, one common classification is distinguishing radioactive isotopes from stable isotopes. Radioactive isotopes are isotopes that will decay into a different isotope. For example, 3H (tritium) is a radioactive isotope of hydrogen. It decays into 3He
with a half-life of ~12.3 years. By comparison, stable isotopes are
more stable, decaying much more slowly and having much longer
half-lives. Examples of stable isotopes are 86Sr and 87Sr. These isotopes of strontium
have half-lives on the order of billions of years or are unmeasured
because of how stable they are. On timescales that geologists and
environmental scientists investigate, these isotopes are stable. Both of
these types of isotopes are useful to scientists. Radioactive isotopes
are generally more useful on shorter timescales, such as investigating
modern circulation of the ocean using 14C, while stable
isotopes are generally more useful on longer timescales, such as
investigating differences in river flow with strontium stable isotopes.
These isotopes are used as tracers to study various phenomena of
interest. These tracers have a certain distribution spatially, and so
scientists need to deconvolve the different processes that affect these
tracer distributions. One way tracer distributions are set is by
conservative mixing. In conservative mixing, the amount of the tracer is
conserved. An example of this is mixing two water masses with different salinities.
The salt from the saltier water mass moves to the less salty water
mass, keeping the total amount of salinity constant. This way of mixing
tracers is very important, giving a baseline of what value of a tracer
one should expect. The value of a tracer as a point is expected to be an
average value of the sources that flow into that region. Deviations
from this are indicative of other processes. These can be called
nonconservative mixing, where there are other processes that do not
conserve the amount of tracer. An example of this is 𝛿14C. This mixes between water masses, but it also decays over time, reducing the amount of 14C in the region.
Useful Elements
The most used environmental isotopes are:
Ocean Circulation
One topic that environmental isotopes are used to study is the
circulation of the ocean. Treating the ocean as a box is only useful in
some studies; in depth consideration of the oceans in general circulation models
(GCM's) requires knowing how the ocean circulates. This leads to an
understanding of how the oceans (along with the atmosphere) transfer
heat from the tropics to the poles. This also helps deconvolve
circulation effects from other phenomena that affect certain tracers
such as radioactive and biological processes.
A
summary of the path of the thermohaline circulation. Blue paths
represent deep-water currents, while red paths represent surface
currents.
Using rudimentary observation techniques, the circulation of the surface ocean can be determined. In the Atlantic basin, surface waters flow from the south towards the north in general, while also creating gyres in the northern and southern Atlantic. In the Pacific Ocean,
the gyres still form, but there is comparatively very little large
scale meridional (North-South) movement. For deep waters, there are two
areas where density causes waters to sink into the deep ocean. These are
in the North Atlantic and the Antarctic. The deep water masses formed
are North Atlantic Deep Water (NADW) and Antarctic Bottom Water
(AABW). Deep waters are mixtures of these two waters, and understanding
how waters are composed of these two water masses can tell us about how
water masses move around in the deep ocean.
This can be investigated with environmental isotopes, including 14C. 14C is predominantly produced in the upper atmosphere and from nuclear testing, with no major sources or sinks in the ocean. This 14C from the atmosphere becomes oxidized into 14CO2,
allowing it to enter the surface ocean through gas transfer. This is
transferred into the deep ocean through NADW and AABW. In NADW, the 𝛿14C is approximately -60‰, and in AABW, the 𝛿14C
is approximately -160‰. Thus, using conservative mixing of radiocarbon,
the expected amount of radiocarbon in various locations can be
determined using the percent compositions of NADW and AABW at that
location. This can be determined using other tracers, such as phosphate
star or salinity.
Deviations from this expected value are indicative of other processes
that affect the delta ratio of radiocarbon, namely radioactive decay.
This deviation can be converted to a time, giving the age of the water
at that location. Doing this over the world's ocean can yield a
circulation pattern of the ocean and the rate at which water flow
through the deep ocean. Using this circulation in conjunction with the
surface circulation allows scientists to understand the energy balance
of the world. Warmer surface waters flow northward while colder deep
waters flow southward, leading to net heat transfer towards the pole.
Paleoclimate
Isotopes are also used to study paleoclimate.
This is the study of how climate was in the past, from hundreds of
years ago to hundreds of thousands of years ago. The only records of
these times that we have are buried in rocks, sediments, biological shells, stalagmites and stalactites,
etc. The isotope ratios in these samples were affected by the
temperature, salinity, circulation of the ocean, precipitation, etc. of
the climate at the time, causing a measurable change from the standards
for isotope measurements. This is how climate information is encoded in
these geological formations. Some of the many isotopes useful for
environmental science are discussed below.
Delta O18
One useful isotope for reconstructing past climates is oxygen-18. It is another stable isotope of oxygen along with oxygen-16, and its incorporation into water and carbon dioxide/carbonate
molecules is strongly temperature dependent. Higher temperature implies
more incorporation of oxygen-18, and vice versa. Thus, the ratio of 18O/16O can tell something about temperature. For water, the isotope ratio standard is Vienna Standard Mean Ocean Water, and for carbonates, the standard is Pee Dee Belemnite. Using ice cores
and sediment cores that record information about the water and shells
from past times, this ratio can tell scientists about the temperature of
those times.
Climate record as reconstructed by
Lisiecki and
Raymo
(2005) showing oscillations in the Earth's temperature over time. These
oscillations have a 41 kyr cycle until about 1.2 million years ago,
switching to a 100 kyr cycle that we see now.
This ratio is used with ice cores to determine the temperature at the
spot in the ice core. Depth in an ice core is proportional to time, and
it is "wiggle-matched" with other records to determine the true time of
the ice at that depth. This can be done by comparing δ18O
in calcium carbonate shells in sediment cores to these records to match
large scale changes in the temperature of the Earth. Once the ice cores
are matched to sediment cores, highly accurate dating methods such as U-series dating
can be used to accurately determine the time of these events. There are
some processes that mix water from different times into the same depth
in the ice core, such as firn production and sloped landscape floes.
Lisiecki and Raymo (2005) used measurements of δ18O in benthic foraminifera
from 57 globally distributed deep sea sediment cores, taken as a proxy
for the total global mass of glacial ice sheets, to reconstruct the
climate for the past five million years.
This record shows oscillations of 2-10 degrees Celsius over this time.
Between 5 million and 1.2 million years ago, these oscillations had a
period of 41,000 years (41 kyr), but about 1.2 million years ago the
period switch to 100 kyr. These changes in global temperature match with
changes in orbital parameters of the Earth's orbit around the Sun.
These are called Milankovitch cycles, and these are related to eccentricity, obliquity (axial tilt), and precession of Earth around its axis. These correspond to cycles with periods of 100 kyr, 40 kyr, and 20 kyr.
δ18O can also be used to investigate smaller scale climate phenomena. Koutavas et al. (2006) used δ18O of G. ruber foraminifera to study the El Niño–Southern Oscillation (ENSO) and it's variability through the mid-Holocene.[6] By isolating individual foram shells, Koutavas et al. were able to obtain a spread of δ18O
values at a specific depth. Because these forams live for approximately
a month and that the individual forams were from many different months,
clumped together in a small depth range in the coral, the variability
of δ18O was able to be determined. In the eastern Pacific,
where these cores were taken, the primary driver of this variability is
ENSO, making this a record of ENSO variability over the core's time
span. Koutavas et al. found that ENSO was much less variable in the mid
Holocene (~6,000 years ago) than it is currently.
Strontium isotopes
Another
set of environmental isotopes used in paleoclimate is strontium
isotopes. Strontium-86 and strontium-87 are both stable isotopes of
strontium, but strontium-87 is radiogenic, coming from the decay of
rubidium-87. The ratio of these two isotopes depends on the
concentration of rubidium-87 initially and the age of the sample,
assuming that the background concentration of strontium-87 is known.
This is useful because 87Rb is predominantly found in
continental rocks. Particles from these rocks come into the ocean
through weathering by rivers, meaning that this strontium isotope ratio
is related to the weathering ion flux coming from rivers into the ocean.
The background concentration in the ocean for 87Sr/86Sr is 0.709 ± 0.0012.
Because the strontium ratio is recorded in sedimentary records, the
oscillations of this ratio over time can be studied. These oscillations
are related to the riverine input into the oceans or into the local
basin. Richter and Turekian have done work on this, finding that over
glacial-interglacial timescales (105 years), the 87Sr/86Sr ratio varies by 3*10−5.
Decay series of Actinides, including Uranium, Protactinium, Thorium, and Lead
Uranium and related isotopes
Uranium has many radioactive isotopes that continue emitting particles down a decay chain.
Uranium-235 is in one such chain, and decays into protactinium-231 and then into other products. Uranium-238 is in a separate chain, decaying into a series of elements, including thorium-230. Both of these series end up forming lead, either lead-207 from uranium-235 or lead-206 from uranium-238. All of these decays are alpha or beta decays, meaning that they all follow first order rate equations of the form  ,
where λ is the half-life of the isotope in question. This makes it
simple to determine the age of a sample based on the various ratios of
radioactive isotopes that exist.
,
where λ is the half-life of the isotope in question. This makes it
simple to determine the age of a sample based on the various ratios of
radioactive isotopes that exist.
One way uranium isotopes are used is to date rocks from millions to billions of years ago. This is through uranium-lead dating. This technique uses zircon samples and measures the lead content in them. Zircon incorporates uranium and thorium atoms into its crystal structure, but strongly rejects lead.
Thus, the only sources of lead in a zircon crystal are through decay of
uranium and thorium. Both the uranium-235 and uranium-238 series decay
into an isotope of lead. The "half-life" of converting 235U to 207Pb is 710 million years, and the "half-life" of converting 238U to 206Pb
is 4.47 billion years. Because of high resolution mass-spectroscopy,
both chains can be used to date rocks, giving complementary information
about the rocks. The large difference in half-lives makes the technique
robust over long time scales, from on the order of millions of years to
on the order of billions of years.
Another way uranium isotopes are used in environmental science is the ratio of 231Pa/230Th.
These radiogenic isotopes have different uranium parents, but have very
different reactivities in the ocean. The uranium profile in the ocean
is constant because uranium has a very large residence time compared to
the residence time of the ocean. The decay of uranium is thus also
isotropic, but the daughter isotopes react differently. Thorium is
readily scavenged by particles, leading to rapid removal from the ocean
into sediments. By contrast, 231Pa is not as particle-reactive, feeling the circulation of the ocean in small amounts before settling into the sediment. Thus, knowing the decay rates of both isotopes and the fractions of each uranium isotopes, the expected ratio of 231Pa/230Th can be determined, with any deviation from this value being due to circulation. Circulation leads to a higher 231Pa/230Th
ratio downstream and a lower ratio upstream, with the magnitude of the
deviation being related to flow rate. This technique has been used to
quantify the Atlantic Meridional Overturning Circulation (AMOC) during the Last Glacial Maximum (LGM) and during abrupt climate change events in Earth's past, such as Heinrich events and Dansgaard-Oeschger events.
Neodymium
Neodymium isotopes are also used to determine circulation in the ocean. All of the isotopes of neodymium are stable on the timescales of glacial-interglacial cycles, but 143Nd is a daughter of 147Sm, a radioactive isotope in the ocean. Samarium-147 has higher concentrations in mantle rocks vs crust rocks, so areas that receive river inputs from mantle-derived rocks have higher concentrations of 147Sm and 143Nd.
However, these differences are so small, the standard notation of a
delta value are no blunt for it; a more precise epsilon value is used to
describe variations in this ratio of neodymium isotopes. It
is defined
as 
The only major sources of this in the ocean are in the North
Atlantic and in the deep Pacific Ocean. Because one of the end-members
is set in the interior of the ocean, this technique has the potential to
tell us complementary information about paleoclimate compared to all
other ocean tracers that are only set in the surface ocean.