From Wikipedia, the free encyclopedia
|
|
| Classification | Baryon |
|---|---|
| Composition | 1 up quark, 2 down quarks |
| Statistics | Fermionic |
| Interactions | Gravity, weak, strong, electromagnetic |
| Symbol | n, n0, N0 |
| Antiparticle | Antineutron |
| Theorized | Ernest Rutherford[1] (1920) |
| Discovered | James Chadwick[2] (1932) |
| Mass | 1.674927351(74)×10−27 kg[3] 939.565378(21) MeV/c2[3] 1.00866491600(43) u[3] |
| Mean lifetime | 881.5(15) s (free) |
| Electric charge | 0 e 0 C |
| Electric dipole moment | < 2.9×10−26 e⋅cm |
| Electric polarizability | 1.16(15)×10−3 fm3 |
| Magnetic moment | −0.96623647(23)×10−26 J·T−1[3] −1.04187563(25)×10−3 μB[3] −1.91304272(45) μN[3] |
| Magnetic polarizability | 3.7(20)×10−4 fm3 |
| Spin | 1⁄2 |
| Isospin | 1⁄2 |
| Parity | +1 |
| Condensed | I(JP) = 1⁄2(1⁄2+) |
The neutron is a subatomic particle, symbol n or n0, with no net electric charge and a mass slightly larger than that of a proton. Protons and neutrons, each with mass approximately one atomic mass unit, constitute the nucleus of an atom, and they are collectively referred to as "nucleons".[4] Their properties and interactions are described by nuclear physics.
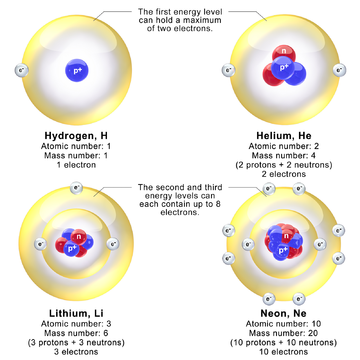
The nucleus consists of a number of protons, or atomic number, with symbol Z, and a number of neutrons, or neutron number, with symbol N. The atomic number defines the chemical properties of the atom, and the neutron number determines the isotope or nuclide.[5] The terms isotope and nuclide are often used synonymously, but they refer to chemical and nuclear properties, respectively. The atomic mass number, symbol A, equals Z+N. For example, carbon has atomic number 6, and its abundant carbon-12 isotope has 6 neutrons, whereas its rare carbon-13 isotope has 7 neutrons. Some elements occur in nature with only one stable isotope, such as fluorine (see stable nuclide). Other elements occur as many stable isotopes, such as tin with ten stable isotopes. Even though it is not a chemical element, the neutron is included in the table of nuclides.[6]
Within the nucleus, protons and neutrons are bound together through the nuclear force, and neutrons are required for the stability of nuclei. Neutrons are produced copiously in nuclear fission and fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes.
The neutron is essential to the production of nuclear power. After the neutron was discovered in 1932,[7] it was quickly realized that neutrons might act to form a nuclear chain reaction. In the 1930s, neutrons were used to produce many different types of nuclear transmutations. When nuclear fission was discovered in 1938,[8] it became clear that, if a fission event produced neutrons, each of these neutrons might cause further fission events, etc., in a cascade known as a chain reaction.[5] These events and findings led to the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity, 1945).
Free neutrons, or individual neutrons free of the nucleus, are effectively a form of ionizing radiation, and as such, are a biological hazard, depending upon dose.[5] A small natural "neutron background" flux of free neutrons exists on Earth, caused by cosmic ray muons, and by the natural radioactivity of spontaneously fissionable elements in the Earth's crust.[9] Dedicated neutron sources like neutron generators, research reactors and spallation sources produce free neutrons for use in irradiation and in neutron scattering experiments.
Description
Neutrons and protons are both nucleons, which are attracted and bound together by the nuclear force to form atomic nuclei. The nucleus of the most common isotope of the hydrogen atom (with the chemical symbol "H") is a lone proton. The nuclei of the heavy hydrogen isotopes deuterium and tritium contain one proton bound to one and two neutrons, respectively. All other types of atomic nuclei are composed of two or more protons and various numbers of neutrons. The most common nuclide of the common chemical element lead (Pb) has 82 protons and 126 neutrons, for example.The free neutron has a mass of about 1.675×10−27 kg (equivalent to 939.6 MeV/c2, or 1.0087 u).[3] The neutron has a mean square radius of about 0.8×10−15 m, or 0.8 fm, and it is a spin-½ fermion.[10] The neutron has a magnetic moment with a negative value, because its orientation is opposite to the neutron's spin.[11] The neutron's magnetic moment causes its motion to be influenced by magnetic fields. Although the neutron has no net electric charge, it does have a slight distribution of charge within it. With its positive electric charge, the proton is directly influenced by electric fields, whereas the response of the neutron to this force is much weaker.
Free neutrons are unstable, having a mean lifetime of just under 15 minutes (881.5±1.5 s) from a radioactive decay known as beta decay.[12] This decay is possible since the mass of the neutron is slightly greater than the proton; the free proton is stable. Neutrons or protons bound in a nucleus can be stable or unstable, depending on the nuclide. Beta decay, in which neutrons decay to protons, or vice versa, is governed by the weak force, and it requires the emission or absorption of electrons and neutrinos, or their antiparticles.
Nucleons behave almost identically under the influence of the nuclear force within the nucleus. The concept of isospin, in which the proton and neutron are viewed as two quantum states of the same particle, is used to model the interactions of nucleons by the nuclear or weak forces. Because of the strength of the nuclear force at short distances, the binding energy of nucleons is more than seven orders of magnitude larger than the electromagnetic energy binding electrons in atoms. Nuclear reactions (such as nuclear fission) therefore have an energy density that is more than ten million times that of chemical reactions. Because of the mass–energy equivalence, nuclear binding energies add or subtract from the mass of nuclei. Ultimately, the ability of the nuclear force to store energy arising from the electromagnetic repulsion of nuclear components is the basis for most of the energy that makes nuclear reactors or bombs possible. In nuclear fission, the absorption of a neutron by a heavy nuclide (e.g., uranium-235) causes the nuclide to become unstable and break into light nuclides and additional neutrons. The positively charged light nuclides then repel, releasing electromagnetic potential energy.
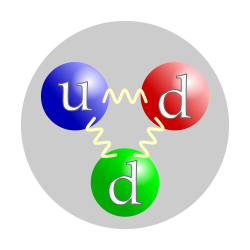
The neutron is classified as a hadron, since it is composed of quarks, and as a baryon, since it is composed of three quarks.[13] The finite size of the neutron and its magnetic moment indicate the neutron is a composite, rather than elementary, particle. The neutron consists of two down quarks with charge −⅓ e and one up quark with charge +⅔ e, although this simple model belies the complexities of the Standard Model for nuclei.[14] The masses of the three quarks sum to only about 12 MeV/c2, whereas the neutron's mass is about 940 MeV/c2, for example.[14] Like the proton, the quarks of the neutron are held together by the strong force, mediated by gluons.[15] The nuclear force results from secondary effects of the more fundamental strong force.
Discovery
The story of the discovery of the neutron and its properties is central to the extraordinary developments in atomic physics that occurred in the first half of the 20th century, leading ultimately to the atomic bomb in 1945. The century began with Ernest Rutherford and Thomas Royds proving that alpha radiation is helium ions in 1908[16][17] and Rutherford's model for the atom in 1911,[18] in which atoms have their mass and positive charge concentrated in a very small nucleus.[19] The essential nature of the atomic nucleus was established with the discovery of the neutron in 1932. By mid-century, these discoveries and subsequent developments had ushered in the atomic age.Rutherford atom
The 1911 Rutherford model was that the atom was made up of a massive central positive charge of small spatial extent surrounded by a larger cloud of negatively charged electrons. This model had been developed from the extraordinary finding that alpha particles were on occasion scattered to high angle when passing through gold foil, indicating the alpha particles were occasionally reflecting from a small, but dense, component of atoms. Rutherford and others noted the disparity between the atomic number of an atom, or number of positive charges, and its mass computed in atomic mass units. The atomic number of an atom is usually about half its atomic mass. In 1920 Rutherford suggested that the disparity could be explained by the existence of a neutrally charged particle within the atomic nucleus.[20] Since at the time no such particle was known to exist, yet the mass of such a particle had to be about equal to that of the proton, Rutherford considered the required particle to be a neutral double consisting of an electron closely orbiting a proton.[20] The mass of protons is about 1800 times greater than that of electrons.There were other motivations for the proton–electron model. As noted by Rutherford at the time, "We have strong reason for believing that the nuclei of atoms contain electrons as well as positively charged bodies...",[20] namely, it was known that beta radiation was electrons emitted from the nucleus.
Rutherford called these uncharged particles neutrons, apparently from the Latin root for neutral and the Greek ending -on (by imitation of electron and proton).[21][22] References to the word neutron in connection with the atom can be found in the literature as early as 1899, however.[23]
Problems of the nuclear electrons hypothesis
Throughout the 1920s, physicists assumed that the atomic nucleus was composed of protons and "nuclear electrons"[24][25] but there were obvious problems. Under this hypothesis, the nitrogen-14 (14N) nucleus, would be composed of 14 protons and 7 electrons so that it would have a net charge of +7 elementary charge units and a mass of 14 atomic mass units. The nucleus was also orbited by another 7 electrons, termed "external electrons" by Rutherford,[20] to complete the 14N atom. The Rutherford model was very influential, however, motivating the Bohr model for electrons orbiting the nucleus in 1913 and eventually leading to quantum mechanics by the mid-1920s.By about 1930 it was generally recognized that it was difficult to reconcile the proton–electron model for nuclei with the Heisenberg uncertainty relation of quantum mechanics.[26][27] This relation, Δx⋅Δp ≥ ½ħ, implies that an electron confined to a region the size of an atomic nucleus has an expected kinetic energy of 10–100 MeV.[27][28][29] This energy is larger than the binding energy of nucleons and larger than the observed energy of beta particles emitted from the nucleus.[27] While these considerations did not "prove" an electron could not exist in the nucleus, they were challenging for physicists to interpret.
The Klein paradox,[30] discovered by Oskar Klein in 1928, presented further quantum mechanical objections to the notion of an electron confined within a nucleus.[26] Derived from the Dirac equation, this clear and precise paradox showed that a high-energy electron approaching a potential barrier has a high probability of passing through the barrier, or escaping, by transforming to a particle of negative mass. Apparently, an electron could not be confined within a nucleus by any potential well. The meaning of this paradox was intensely debated at the time.[26]
Observations of the energy levels of atoms and molecules were inconsistent with the nuclear spin expected from proton–electron hypothesis. Molecular spectroscopy of dinitrogen (14N2) showed that transitions originating from even rotational levels are more intense than those from odd levels, hence the even levels are more populated. According to quantum mechanics and the Pauli exclusion principle, the spin of the 14N nucleus is therefore an integer multiple of ħ (the reduced Planck constant).[31][32] Both protons and electrons carry an intrinsic spin of ½ ħ, and there is no way to arrange an odd number (14 protons + 7 electrons = 21) of spins ±½ ħ to give a spin of 1 ħ.
The observed hyperfine structure of atomic spectra was inconsistent to the proton–electron hypothesis. This structure is caused by the influence of the nucleus on the dynamics of orbiting electrons. The magnetic moments of supposed "nuclear electrons" should produce hyperfine spectral line splittings similar to the Zeeman effect , but no such effects were observed.[26] This contradiction was somewhat mysterious,[24] until it was realized that there are no individual nuclear electrons in the nucleus.
Discovery of the neutron
In 1931, Walther Bothe and Herbert Becker in Giessen, Germany found that if the very energetic alpha particles emitted from polonium fell on certain light elements, specifically beryllium, boron, or lithium, an unusually penetrating radiation was produced. Since this radiation was not influenced by an electric field (neutrons have no charge), it was thought to be gamma radiation. The radiation was more penetrating than any gamma rays known, and the details of experimental results were difficult to interpret.[33][34] The following year Irène Joliot-Curie and Frédéric Joliot in Paris showed that if this unknown radiation fell on paraffin, or any other hydrogen-containing compound, it ejected protons of very high energy.[35] This observation was not in itself inconsistent with the assumed gamma ray nature of the new radiation, but detailed quantitative analysis of the data became increasingly difficult to reconcile with such a hypothesis. In Rome, the young physicist Ettore Majorana suggested that the manner in which the new radiation interacted with protons required a new neutral particle.[36]On hearing of the Paris results in 1932, neither Rutherford nor James Chadwick at the Cavendish Laboratory in Cambridge were convinced by the gamma ray hypothesis.[24] Chadwick had searched for Rutherford's neutron by several experiments throughout the 1920s without success. Chadwick quickly performed a series of experiments showing that the gamma ray hypothesis was untenable. He repeated the creation of the radiation using beryllium, used better approaches to detection, and aimed the radiation at paraffin following the Paris experiment. Paraffin is high in hydrogen content, hence offers a target dense with protons; since neutrons and protons have almost equal mass, protons scatter energetically from neutrons. Chadwick measured the range of these protons, and also measured how the new radiation impacted the atoms of various gases.[37] He found that the new radiation consisted of not gamma rays, but uncharged particles with about the same mass as the proton; these particles were neutrons.[7][38] Chadwick won the Nobel Prize in Physics for this discovery in 1935.[2]
Proton–neutron model of the nucleus
Given the problems of the proton–electron model,[24][25] it was quickly accepted that the atomic nucleus is composed of protons and neutrons. Within months after the discovery of the neutron, Werner Heisenberg[39][40][41] and Dmitri Ivanenko[42] had proposed proton–neutron models for the nucleus.[43] Heisenberg's landmark papers approached the description of protons and neutrons in the nucleus through quantum mechanics. While Heisenberg's theory for protons and neutrons in the nucleus was a "major step toward understanding the nucleus as a quantum mechanical system,"[44] he still assumed the presence of nuclear electrons. In particular, Heisenberg assumed the neutron was a proton–electron composite, for which there is no quantum mechanical explanation. Heisenberg had no explanation for how lightweight electrons could be bound within the nucleus. Heisenberg introduced the first theory of nuclear exchange forces that bind the nucleons. He considered protons and neutrons to be different quantum states of the same particle, i.e., nucleons distinguished by the value of their nuclear isospin quantum numbers.
The proton–neutron model explained the puzzle of dinitrogen. When 14N was proposed to consist of 3 pairs each of protons and neutrons, with an additional unpaired neutron and proton each contributing a spin of 1⁄2 ħ in the same direction for a total spin of 1 ħ, the model became viable. [45] [46][47] Soon, neutrons were used to naturally explain spin differences in many different nuclides in the same way.
If the proton–neutron model for the nucleus resolved many issues, it highlighted the problem of explaining the origins of beta radiation. No existing theory could account for how electrons could emanate from the nucleus. In 1934, Enrico Fermi published his classic paper describing the process of beta decay, in which the neutron decays to a proton by creating an electron and a (as yet undiscovered) neutrino.[48] The paper employed the analogy that photons, or electromagnetic radiation, were similarly created and destroyed in atomic processes. Ivanenko had suggested a similar analogy in 1932.[49][45] Fermi's theory requires the neutron to be a spin-½ particle. The theory preserved the principle of conservation of energy, which had been thrown into question by the continuous energy distribution of beta particles. The basic theory for beta decay proposed by Fermi was the first to show how particles could be created and destroyed. It established a general, basic theory for the interaction of particles by weak or strong forces.[48] While this influential paper has stood the test of time, the ideas within it were so new that when it was first submitted to the journal Nature in 1933 it was rejected as being too speculative.[44]
The question of whether the neutron was a composite particle of a proton and an electron persisted for a few years after its discovery.[50][51] The issue was a legacy of the prevailing view from the 1920s that the only elementary particles were the proton and electron. The nature of the neutron was a primary topic of discussion at the 7th Solvay Conference held in October 1933, attended by Heisenberg, Niels Bohr, Lise Meitner, Ernest Lawrence, Fermi, Chadwick, and others.[44][52] The primary question was the mass of the neutron relative to the proton. If the neutron's mass was less than the combined masses of a proton and a electron (1.0078 u), then the neutron could be a composite because of the mass defect from the binding energy. If greater than the combined masses, then the neutron was elementary. The question was challenging to answer because the electron's mass is only 0.05% of the proton's, hence precise measurements were required.
The difficulty of making the measurement is illustrated by the wide ranging values for the mass of the neutron obtained from 1932-1934. The accepted value today is 1.00866 u. In Chadwick's 1934 paper reporting on the discovery, he estimated the mass of the neutron to be between 1.005 u and 1.008 u.[53] By bombarding boron with alpha particles, Frédéric and Irène Joliot-Curie obtained a high value of 1.012 u, while Ernest Lawrence's team at the University of California measured the small value 1.0006 u using their new cyclotron.[54] In support of Fermi's theory and the neutron as an elementary particle, in 1935 Chadwick and his doctoral student Maurice Goldhaber, reported the first accurate measurement of the mass of the neutron. They used the 2.6 MeV gamma rays of 208Tl (then known as thorium C") to photodisintegrate deuterium, and used the energies of the resulting proton and neutron to infer the neutron's mass. Chadwick and Goldhaber found the neutron's mass to be slightly greater than the mass of the proton (1.0084 u or 1.0090 u, depending on precise values used for the proton and deuteron masses), and therefore predicted that an unbound neutron is unstable and would undergo beta decay.[55][56] The mass of the neutron was too large to be a proton-electron composite.[53]
Neutron physics in the 1930s
Soon after the discovery of the neutron, indirect evidence suggested the neutron had an unexpected non-zero value for its magnetic moment. Attempts to measure the neutron's magnetic moment originated with the discovery by Otto Stern in 1933 in Hamburg that the proton had an anomalously large magnetic moment.[57][58] By 1934 groups led by Stern, now in Pittsburgh, and I. I. Rabi in New York had independently deduced that the magnetic moment of the neutron was negative and unexpectedly large by measuring the magnetic moments of the proton and deuteron.[59][60][61] [51][62] Values for the magnetic moment of the neutron were also determined by Robert Bacher[63] at Ann Arbor (1933) and I.Y. Tamm and S.A. Altshuler[64][51] (1934) in the Soviet Union from studies of the hyperfine structure of atomic spectra. By the late 1930's accurate values for the magnetic moment of the neutron had been deduced by the Rabi group using measurements employing newly-developed nuclear magnetic resonance techniques.[62] The large value for the proton's magnetic moment and the inferred negative value for the neutron's magnetic moment were unexpected and raised many questions.[51]The discovery of the neutron immediately gave scientists a new tool for probing the properties of atomic nuclei. Alpha particles had been used over the previous decades in scattering experiments, but such particles, which are helium nuclei, have +2 charge. This charge makes it difficult for alpha particles to overcome the Coulomb repulsive force and interact directly with the nuclei of atoms. Since neutrons have no electric charge, they do not have to overcome this force to interact with nuclei. Almost coincident with its discovery, neutrons were used by Norman Feather, Chadwick's colleague and protege, in scattering experiments with nitrogen.[65] Feather was able to show that neutrons interacting with nitrogen nuclei scattered to protons or induced nitrogen to disintegrate to form boron with the emission of an alpha particle. Feather was therefore the first to show that neutrons produce nuclear disintegrations.
In Rome Enrico Fermi bombarded heavier elements with neutrons and found them to be radioactive. By 1934 Fermi had used neutrons to induce radioactivity in 22 different elements, many of these elements of high atomic number. Noticing that other experiments with neutrons at his laboratory seemed to work better on a wooden table than a marble table, Fermi suspected that the protons of the wood were slowing the neutrons and so increasing the chance for the neutron to interact with nuclei. Fermi therefore passed neutrons through paraffin wax to slow them and found that the radioactivity of bombarded elements increased by a hundredfold. The cross section for interaction with nuclei is much larger for slow neutrons than for fast neutrons. In 1938 Fermi received the Nobel Prize in Physics "for his demonstrations of the existence of new radioactive elements produced by neutron irradiation, and for his related discovery of nuclear reactions brought about by slow neutrons".[66]
Jointly with Lise Meitner and his pupil and assistant Fritz Strassmann, Otto Hahn furthered the research begun by Fermi and his team when he bombarded uranium with neutrons at his laboratory in Berlin. Between 1934 and 1938, Hahn, Meitner, and Strassmann found a great number of radioactive transmutation products from these experiments, all of which they regarded as transuranic.[67] The decisive experiment on 16–17 December 1938 (the celebrated "radium–barium–mesothorium–fractionation") produced puzzling results: the three isotopes consistently behaved not as radium, but as barium.[68] By January 1939 Hahn had concluded that he was seeing light platinoids, barium, lanthanum, and cerium. Hahn and his collaborators had observed nuclear fission, or the fractionation of uranium nuclei into light elements, induced by neutron bombardment. In their second publication on nuclear fission, Hahn and Strassmann predicted the existence and liberation of additional neutrons during the fission process.[69] Frédéric Joliot and his team proved this phenomena to be a chain reaction in March 1939. In 1945 Hahn received the 1944 Nobel Prize in Chemistry "for his discovery of the fission of heavy atomic nuclei." [70][71][72]
The discovery of nuclear fission at the end of 1938 marked a shift in the centers of nuclear research from Europe to the United States. Large numbers of scientists were migrating to the United States to escape the troubles in Europe and the looming war (See Jewish scientists and the Manhattan Project). The new centers of nuclear research were the universities in the United States, particularly Columbia University in New York and the University of Chicago where Enrico Fermi had relocated, and a new research facility at Los Alamos, New Mexico beginning in 1942, the new home of the Manhattan project.
Beta decay and the stability of the nucleus
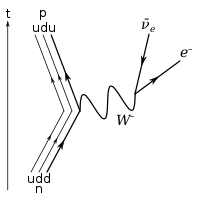
The Feynman diagram for beta decay of a neutron into a proton, electron, and electron antineutrino via an intermediate heavy W boson
Under the Standard Model of particle physics, the only possible decay mode for the neutron that conserves baryon number is for one of the neutron's quarks to change flavour via the weak interaction. The decay of one of the neutron's down quarks into a lighter up quark can be achieved by the emission of a W boson. By this process, the Standard Model description of beta decay, the neutron decays into a proton (which contains one down and two up quarks), an electron, and an electron antineutrino.
Since interacting protons have a mutual electromagnetic repulsion that is stronger than their attractive nuclear interaction, neutrons are a necessary constituent of any atomic nucleus that contains more than one proton (see diproton and neutron–proton ratio).[73] Neutrons bind with protons and one another in the nucleus via the nuclear force, effectively moderating the repulsive forces between the protons and stabilizing the nucleus.
Free neutron decay
Outside the nucleus, free neutrons are unstable and have a mean lifetime of 881.5±1.5 s (about 14 minutes, 42 seconds); therefore the half-life for this process (which differs from the mean lifetime by a factor of ln(2) = 0.693) is 611.0±1.0 s (about 10 minutes, 11 seconds).[12] Beta decay of the neutron, described above, can be denoted by the radioactive decay:[74]- n0 → p+ + e− + ν
e
e denote the proton, electron and electron antineutrino, respectively. For the free neutron the decay energy for this process (based on the masses of the neutron, proton, and electron) is 0.782343 MeV. The maximal energy of the beta decay electron (in the process wherein the neutrino receives a vanishingly small amount of kinetic energy) has been measured at 0.782 ± .013 MeV.[75] The latter number is not well-enough measured to determine the comparatively tiny rest mass of the neutrino (which must in theory be subtracted from the maximal electron kinetic energy) as well as neutrino mass is constrained by many other methods.
A small fraction (about one in 1000) of free neutrons decay with the same products, but add an extra particle in the form of an emitted gamma ray:
- n0 → p+ + e− + ν
e + γ
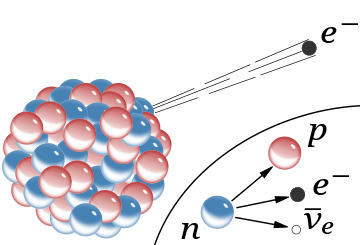
A schematic of the nucleus of an atom indicating β− radiation, the emission of a fast electron from the nucleus (the accompanying antineutrino is omitted). In the Rutherford model for the nucleus, red spheres were protons with positive charge and blue spheres were protons tightly bound to an electron with no net charge.
The inset shows beta decay of a free neutron as it is understood today; an electron and antineutrino are created in this process.
The inset shows beta decay of a free neutron as it is understood today; an electron and antineutrino are created in this process.
A very small minority of neutron decays (about four per million) are so-called "two-body (neutron) decays", in which a proton, electron and antineutrino are produced as usual, but the electron fails to gain the 13.6 eV necessary energy to escape the proton, and therefore simply remains bound to it, as a neutral hydrogen atom (one of the "two bodies"). In this type of free neutron decay, in essence all of the neutron decay energy is carried off by the antineutrino (the other "body").
The transformation of a free proton to a neutron (plus a positron and a neutrino) is energetically impossible, since a free neutron has a greater mass than a free proton.
Bound neutron decay
While a free neutron has a half life of about 10.2 min, most neutrons within nuclei are stable. According to the nuclear shell model, the protons and neutrons of a nuclide are a quantum mechanical system organized into discrete energy levels with unique quantum numbers. For a neutron to decay, the resulting proton requires an available state at lower energy than the initial neutron state.
In stable nuclei the possible lower energy states are all filled, meaning they are each occupied by two protons with spin up and spin down. The Pauli exclusion principle therefore disallows the decay of a neutron to a proton within stable nuclei. The situation is similar to electrons of an atom, where electrons have distinct atomic orbitals and are prevented from decaying to lower energy states, with the emission of a photon, by the exclusion principle.Neutrons in unstable nuclei can decay by beta decay as described above. In this case, an energetically allowed quantum state is available for the proton resulting from the decay. One example of this decay is carbon-14 (6 protons, 8 neutrons) that decays to nitrogen-14 (7 protons, 7 neutrons) with a half-life of about 5,730 years.
Inside a nucleus, a proton can transform into a neutron via inverse beta decay, if an energetically allowed quantum state is available for the neutron. This transformation occurs by emission of an antielectron (also called positron) and an electron neutrino:
The simplified classical view of the neutron's charge distribution also "explains" the fact that the neutron magnetic dipole points in the opposite direction from its spin angular momentum vector (as compared to the proton). This gives the neutron, in effect, a magnetic moment which resembles a negatively charged particle. This can be reconciled classically with a neutral neutron composed of a charge distribution in which the negative sub-parts of the neutron have a larger average radius of distribution, and therefore contribute more to the particle's magnetic dipole moment, than do the positive parts that are, on average, nearer the core.
Bd ) of the single 0.7822 MeV gamma photon emitted when neutrons are captured by protons (this is exothermic and happens with zero-energy neutrons), plus the small recoil kinetic energy (Erd ) of the deuteron (about 0.06% of the total energy).
The dineutron is another hypothetical particle. In 2012, Artemis Spyrou from Michigan State University and coworkers reported that they observed, for the first time, the dineutron emission in the decay of 16Be. The dineutron character is evidenced by a small emission angle between the two neutrons. The authors measured the two-neutron separation energy to be 1.35(10) MeV, in good agreement with shell model calculations, using standard interactions for this mass region.[87]
The extreme pressure inside a neutron star may deform the neutrons into a cubic symmetry, allowing tighter packing of neutrons.[88]
Detectors relying on elastic scattering are called fast neutron detectors. Recoiling nuclei can ionize and excite further atoms through collisions. Charge and/or scintillation light produced in this way can be collected to produce a detected signal. A major challenge in fast neutron detection is discerning such signals from erroneous signals produced by gamma radiation in the same detector.
Fast neutron detectors have the advantage of not requiring a moderator, and therefore being capable of measuring the neutron's energy, time of arrival, and in certain cases direction of incidence.
Natural neutron background. A small natural background flux of free neutrons exists everywhere on Earth. In the atmosphere and deep into the ocean, the "neutron background" is caused by muons produced by cosmic ray interaction with the atmosphere. These high energy muons are capable of penetration to considerable depths in water and soil. There, in striking atomic nuclei, among other reactions they induce spallation reactions in which a neutron is liberated from the nucleus. Within the Earth's crust a second source is neutrons produced primarily by spontaneous fission of uranium and thorium present in crustal minerals. The neutron background is not strong enough to be a biological hazard, but it is of importance to very high resolution particle detectors that are looking for very rare events, such as (hypothesized) interactions that might be caused by particles of dark matter.[9]
Even stronger neutron background radiation is produced at the surface of Mars, where the atmosphere is thick enough to generate neutrons from cosmic ray muon production and neutron-spallation, but not thick enough to provide significant protection from the neutrons produced. These neutrons not only produce a Martian surface neutron radiation hazard from direct downward-going neutron radiation but may also produce a significant hazard from reflection of neutrons from the Martian surface, which will produce reflected neutron radiation penetrating upward into a Martian craft or habitat from the floor.[89]
Sources of neutrons for research. These include certain types of radioactive decay (spontaneous fission and neutron emission), and from certain nuclear reactions. Convenient nuclear reactions include tabletop reactions such as natural alpha and gamma bombardment of certain nuclides, often beryllium or deuterium, and induced nuclear fission, such as occurs in nuclear reactors. In addition, high-energy nuclear reactions (such as occur in cosmic radiation showers or accelerator collisions) also produce neutrons from disintigration of target nuclei. Small (tabletop) particle accelerators optimized to produce free neutrons in this way, are called neutron generators.
In practice, the most commonly used small laboratory sources of neutrons use radioactive decay to power neutron production. One noted neutron-producing radioisotope, californium-252 decays (half-life 2.65 years) by spontaneous fission 3% of the time with production of 3.7 neutrons per fission, and is used alone as a neutron source from this process. Nuclear reaction sources (that involve two materials) powered by radioisotopes use an alpha decay source plus a beryllium target, or else a source of high-energy gamma radiation from a source that undergoes beta decay followed by gamma decay, which produces photoneutrons on interaction of the high energy gamma ray with ordinary stable beryllium, or else with the deuterium in heavy water. A popular source of the latter type is radioactive antimony-124 plus beryllium, a system with a half-life of 60.9 days, which can be constructed from natural antimony (which is 42.8% stable antimony-123) by activating it with neutrons in a nuclear reactor, then transported to where the neutron source is needed.[90]
Nuclear fission reactors naturally produce free neutrons; their role is to sustain the energy-producing chain reaction. The intense neutron radiation can also be used to produce various radioisotopes through the process of neutron activation, which is a type of neutron capture.
Experimental nuclear fusion reactors produce free neutrons as a waste product. However, it is these neutrons that possess most of the energy, and converting that energy to a useful form has proved a difficult engineering challenge. Fusion reactors that generate neutrons are likely to create radioactive waste, but the waste is composed of neutron-activated lighter isotopes, which have relatively short (50–100 years) decay periods as compared to typical half-lives of 10,000 years[citation needed] for fission waste, which is long due primarily to the long half-life of alpha-emitting transuranic actinides.[91]
The neutron's lack of total electric charge makes it difficult to steer or accelerate them. Charged particles can be accelerated, decelerated, or deflected by electric or magnetic fields. These methods have little effect on neutrons. However, some effects may be attained by use of inhomogeneous magnetic fields because of the neutron's magnetic moment. Neutrons can be controlled by methods that include moderation, reflection, and velocity selection. Thermal neutrons can be polarized by transmission through magnetic materials in a method analogous to the Faraday effect for photons. Cold neutrons of wavelengths of 6–7 angstroms can be produced in beams of a high degree of polarization, by use of magnetic mirrors and magnetized interference filters.[92]
Cold, thermal and hot neutron radiation is commonly employed in neutron scattering facilities, where the radiation is used in a similar way one uses X-rays for the analysis of condensed matter. Neutrons are complementary to the latter in terms of atomic contrasts by different scattering cross sections; sensitivity to magnetism; energy range for inelastic neutron spectroscopy; and deep penetration into matter.
The development of "neutron lenses" based on total internal reflection within hollow glass capillary tubes or by reflection from dimpled aluminum plates has driven ongoing research into neutron microscopy and neutron/gamma ray tomography.[93][94][95]
A major use of neutrons is to excite delayed and prompt gamma rays from elements in materials. This forms the basis of neutron activation analysis (NAA) and prompt gamma neutron activation analysis (PGNAA). NAA is most often used to analyze small samples of materials in a nuclear reactor whilst PGNAA is most often used to analyze subterranean rocks around bore holes and industrial bulk materials on conveyor belts.
Another use of neutron emitters is the detection of light nuclei, in particular the hydrogen found in water molecules. When a fast neutron collides with a light nucleus, it loses a large fraction of its energy. By measuring the rate at which slow neutrons return to the probe after reflecting off of hydrogen nuclei, a neutron probe may determine the water content in soil.
Fast neutron therapy utilizes high energy neutrons typically greater than 20 MeV to treat cancer. Radiation therapy of cancers is based upon the biological response of cells to ionizing radiation. If radiation is delivered in small sessions to damage cancerous areas, normal tissue will have time to repair itself, while tumor cells often cannot.[96] Neutron radiation can deliver energy to a cancerous region at a rate an order of magnitude larger than gamma radiation[97]
Beams of low energy neutrons are used in boron capture therapy to treat cancer. In boron capture therapy, the patient is given a drug that contains boron and that preferentially accumulates in the tumor to be targeted. The tumor is then bombarded with very low energy neutrons (although often higher than thermal energy) which are captured by the boron-10 isotope in the boron, which produces an excited state of boron-11 that then decays to produce lithium-7 and an alpha particle that have sufficient energy to kill the malignant cell, but insufficient range to damage nearby cells. For such a therapy to be applied to the treatment of cancer, a neutron source having an intensity of the order of billion (109) neutrons per second per cm2 is preferred. Such fluxes require a research nuclear reactor.
Hydrogen-rich ordinary water affects neutron absorption in nuclear fission reactors: Usually, neutrons are so strongly absorbed by normal water that fuel enrichment with fissionable isotope is required. The deuterium in heavy water has a very much lower absorption affinity for neutrons than does protium (normal light hydrogen). Deuterium is, therefore, used in CANDU-type reactors, in order to slow (moderate) neutron velocity, to increase the probability of nuclear fission compared to neutron capture.
In many substances, thermal neutron reactions show a much larger effective cross-section than reactions involving faster neutrons, and thermal neutrons can therefore be absorbed more readily (i.e., with higher probability) by any atomic nuclei that they collide with, creating a heavier — and often unstable — isotope of the chemical element as a result.
Most fission reactors use a neutron moderator to slow down, or thermalize the neutrons that are emitted by nuclear fission so that they are more easily captured, causing further fission. Others, called fast breeder reactors, use fission energy neutrons directly.
Cold neutrons are particularly valuable for neutron scattering experiments.[citation needed]
Inside a nucleus, a proton can transform into a neutron via inverse beta decay, if an energetically allowed quantum state is available for the neutron. This transformation occurs by emission of an antielectron (also called positron) and an electron neutrino:
- p+ → n0 + e+ + ν
e
- p+ + e− → n0 + ν
e
Competition of beta decay types
Three types of beta decay in competition are illustrated by the single isotope copper-64 (29 protons, 35 neutrons), which has a half-life of about 12.7 hours. This isotope has one unpaired proton and one unpaired neutron, so either the proton or the neutron can decay. This particular nuclide (though not all nuclides in this situation) is almost equally likely to decay through proton decay by positron emission (18%) or electron capture (43%), as through neutron decay by electron emission (39%).Intrinsic properties
Electric charge
The total electric charge of the neutron is 0 e. This zero value has been tested experimentally, and the present experimental limit for the charge of the neutron is −2(8)×10−22 e,[76] or −3(13)×10−41 C. This value is consistent with zero, given the experimental uncertainties (indicated in parentheses). By comparison, the charge of the proton is, of course, +1 e.Electric dipole moment
The Standard Model of particle physics predicts a tiny separation of positive and negative charge within the neutron leading to a permanent electric dipole moment.[77] The predicted value is, however, well below the current sensitivity of experiments. From several unsolved puzzles in particle physics, it is clear that the Standard Model is not the final and full description of all particles and their interactions. New theories going beyond the Standard Model generally lead to much larger predictions for the electric dipole moment of the neutron. Currently, there are at least four experiments trying to measure for the first time a finite neutron electric dipole moment, including:- Cryogenic neutron EDM experiment being set up at the Institut Laue–Langevin[78]
- nEDM experiment under construction at the new UCN source at the Paul Scherrer Institute[79]
- nEDM experiment being envisaged at the Spallation Neutron Source[80]
- nEDM experiment being built at the Institut Laue–Langevin[81]
Magnetic moment
Even though the neutron is a neutral particle, the magnetic moment of a neutron is not zero. Since the neutron is a neutral particle, it is not affected by electric fields, but with its magnetic moment it is affected by magnetic fields. The magnetic moment of the neutron is an indication of its quark substructure and internal charge distribution.[82] The value for the neutron's magnetic moment was first directly measured by Luis Alvarez and Felix Bloch at Berkeley, California in 1940,[83] using an extension of the magnetic resonance methods developed by Rabi. Alvarez and Bloch determined the magnetic moment of the neutron to be μn = −1.93(2) μN, where μN is the nuclear magneton.Structure and geometry of charge distribution
An article published in 2007 featuring a model-independent analysis concluded that the neutron has a negatively charged exterior, a positively charged middle, and a negative core.[84] In a simplified classical view, the negative "skin" of the neutron assists it to be attracted to the protons with which it interacts in the nucleus. (However, the main attraction between neutrons and protons is via the nuclear force, which does not involve charge.)The simplified classical view of the neutron's charge distribution also "explains" the fact that the neutron magnetic dipole points in the opposite direction from its spin angular momentum vector (as compared to the proton). This gives the neutron, in effect, a magnetic moment which resembles a negatively charged particle. This can be reconciled classically with a neutral neutron composed of a charge distribution in which the negative sub-parts of the neutron have a larger average radius of distribution, and therefore contribute more to the particle's magnetic dipole moment, than do the positive parts that are, on average, nearer the core.
Mass
The mass of a neutron cannot be directly determined by mass spectrometry due to lack of electric charge. However, since the mass of protons and deuterons can be measured by mass spectrometry, the mass of a neutron can be deduced by subtracting proton mass from deuteron mass, with the difference being the mass of the neutron plus the binding energy of deuterium (expressed as a positive emitted energy). The latter can be directly measured by measuring the energy (mn=md−mp+Bd−Erd
- mneutron = 1.008644904(14) u
- mneutron = 939.56563(28) MeV/c2.
Anti-neutron
The antineutron is the antiparticle of the neutron. It was discovered by Bruce Cork in the year 1956, a year after the antiproton was discovered. CPT-symmetry puts strong constraints on the relative properties of particles and antiparticles, so studying antineutrons yields provide stringent tests on CPT-symmetry. The fractional difference in the masses of the neutron and antineutron is (9±6)×10−5. Since the difference is only about two standard deviations away from zero, this does not give any convincing evidence of CPT-violation.[12]Neutron compounds
Dineutrons and tetraneutrons
The existence of stable clusters of 4 neutrons, or tetraneutrons, has been hypothesised by a team led by Francisco-Miguel Marqués at the CNRS Laboratory for Nuclear Physics based on observations of the disintegration of beryllium-14 nuclei. This is particularly interesting because current theory suggests that these clusters should not be stable.The dineutron is another hypothetical particle. In 2012, Artemis Spyrou from Michigan State University and coworkers reported that they observed, for the first time, the dineutron emission in the decay of 16Be. The dineutron character is evidenced by a small emission angle between the two neutrons. The authors measured the two-neutron separation energy to be 1.35(10) MeV, in good agreement with shell model calculations, using standard interactions for this mass region.[87]
Neutronium and neutron stars
At extremely high pressures and temperatures, nucleons and electrons are believed to collapse into bulk neutronic matter, called neutronium. This is presumed to happen in neutron stars.The extreme pressure inside a neutron star may deform the neutrons into a cubic symmetry, allowing tighter packing of neutrons.[88]
Detection
The common means of detecting a charged particle by looking for a track of ionization (such as in a cloud chamber) does not work for neutrons directly. Neutrons that elastically scatter off atoms can create an ionization track that is detectable, but the experiments are not as simple to carry out; other means for detecting neutrons, consisting of allowing them to interact with atomic nuclei, are more commonly used. The commonly used methods to detect neutrons can therefore be categorized according to the nuclear processes relied upon, mainly neutron capture or elastic scattering. A good discussion on neutron detection is found in chapter 14 of the book Radiation Detection and Measurement by Glenn F. Knoll (John Wiley & Sons, 1979).Neutron detection by neutron capture
A common method for detecting neutrons involves converting the energy released from neutron capture reactions into electrical signals. Certain nuclides have a high neutron capture cross section, which is the probability of absorbing a neutron. Upon neutron capture, the compound nucleus emits more easily detectable radiation, for example an alpha particle, which is then detected. The nuclides 3He, 6Li, 10B, 233U, 235U, 237Np and 239Pu are useful for this purpose.Neutron detection by elastic scattering
Neutrons can elastically scatter off nuclei, causing the struck nucleus to recoil. Kinematically, a neutron can transfer more energy to light nuclei such as hydrogen or helium than to heavier nuclei.Detectors relying on elastic scattering are called fast neutron detectors. Recoiling nuclei can ionize and excite further atoms through collisions. Charge and/or scintillation light produced in this way can be collected to produce a detected signal. A major challenge in fast neutron detection is discerning such signals from erroneous signals produced by gamma radiation in the same detector.
Fast neutron detectors have the advantage of not requiring a moderator, and therefore being capable of measuring the neutron's energy, time of arrival, and in certain cases direction of incidence.
Sources and production
Free neutrons are unstable, although they have the longest half-life of any unstable sub-atomic particle by several orders of magnitude. Their half-life is still only about 10 minutes, however, so they can be obtained only from sources that produce them freshly.Natural neutron background. A small natural background flux of free neutrons exists everywhere on Earth. In the atmosphere and deep into the ocean, the "neutron background" is caused by muons produced by cosmic ray interaction with the atmosphere. These high energy muons are capable of penetration to considerable depths in water and soil. There, in striking atomic nuclei, among other reactions they induce spallation reactions in which a neutron is liberated from the nucleus. Within the Earth's crust a second source is neutrons produced primarily by spontaneous fission of uranium and thorium present in crustal minerals. The neutron background is not strong enough to be a biological hazard, but it is of importance to very high resolution particle detectors that are looking for very rare events, such as (hypothesized) interactions that might be caused by particles of dark matter.[9]
Even stronger neutron background radiation is produced at the surface of Mars, where the atmosphere is thick enough to generate neutrons from cosmic ray muon production and neutron-spallation, but not thick enough to provide significant protection from the neutrons produced. These neutrons not only produce a Martian surface neutron radiation hazard from direct downward-going neutron radiation but may also produce a significant hazard from reflection of neutrons from the Martian surface, which will produce reflected neutron radiation penetrating upward into a Martian craft or habitat from the floor.[89]
Sources of neutrons for research. These include certain types of radioactive decay (spontaneous fission and neutron emission), and from certain nuclear reactions. Convenient nuclear reactions include tabletop reactions such as natural alpha and gamma bombardment of certain nuclides, often beryllium or deuterium, and induced nuclear fission, such as occurs in nuclear reactors. In addition, high-energy nuclear reactions (such as occur in cosmic radiation showers or accelerator collisions) also produce neutrons from disintigration of target nuclei. Small (tabletop) particle accelerators optimized to produce free neutrons in this way, are called neutron generators.
In practice, the most commonly used small laboratory sources of neutrons use radioactive decay to power neutron production. One noted neutron-producing radioisotope, californium-252 decays (half-life 2.65 years) by spontaneous fission 3% of the time with production of 3.7 neutrons per fission, and is used alone as a neutron source from this process. Nuclear reaction sources (that involve two materials) powered by radioisotopes use an alpha decay source plus a beryllium target, or else a source of high-energy gamma radiation from a source that undergoes beta decay followed by gamma decay, which produces photoneutrons on interaction of the high energy gamma ray with ordinary stable beryllium, or else with the deuterium in heavy water. A popular source of the latter type is radioactive antimony-124 plus beryllium, a system with a half-life of 60.9 days, which can be constructed from natural antimony (which is 42.8% stable antimony-123) by activating it with neutrons in a nuclear reactor, then transported to where the neutron source is needed.[90]
Nuclear fission reactors naturally produce free neutrons; their role is to sustain the energy-producing chain reaction. The intense neutron radiation can also be used to produce various radioisotopes through the process of neutron activation, which is a type of neutron capture.
Experimental nuclear fusion reactors produce free neutrons as a waste product. However, it is these neutrons that possess most of the energy, and converting that energy to a useful form has proved a difficult engineering challenge. Fusion reactors that generate neutrons are likely to create radioactive waste, but the waste is composed of neutron-activated lighter isotopes, which have relatively short (50–100 years) decay periods as compared to typical half-lives of 10,000 years[citation needed] for fission waste, which is long due primarily to the long half-life of alpha-emitting transuranic actinides.[91]
Neutron beams and modification of beams after production
Free neutron beams are obtained from neutron sources by neutron transport. For access to intense neutron sources, researchers must go to a specialist neutron facility that operates a research reactor or a spallation source.The neutron's lack of total electric charge makes it difficult to steer or accelerate them. Charged particles can be accelerated, decelerated, or deflected by electric or magnetic fields. These methods have little effect on neutrons. However, some effects may be attained by use of inhomogeneous magnetic fields because of the neutron's magnetic moment. Neutrons can be controlled by methods that include moderation, reflection, and velocity selection. Thermal neutrons can be polarized by transmission through magnetic materials in a method analogous to the Faraday effect for photons. Cold neutrons of wavelengths of 6–7 angstroms can be produced in beams of a high degree of polarization, by use of magnetic mirrors and magnetized interference filters.[92]
Applications
The neutron plays an important role in many nuclear reactions. For example, neutron capture often results in neutron activation, inducing radioactivity. In particular, knowledge of neutrons and their behavior has been important in the development of nuclear reactors and nuclear weapons. The fissioning of elements like uranium-235 and plutonium-239 is caused by their absorption of neutrons.Cold, thermal and hot neutron radiation is commonly employed in neutron scattering facilities, where the radiation is used in a similar way one uses X-rays for the analysis of condensed matter. Neutrons are complementary to the latter in terms of atomic contrasts by different scattering cross sections; sensitivity to magnetism; energy range for inelastic neutron spectroscopy; and deep penetration into matter.
The development of "neutron lenses" based on total internal reflection within hollow glass capillary tubes or by reflection from dimpled aluminum plates has driven ongoing research into neutron microscopy and neutron/gamma ray tomography.[93][94][95]
A major use of neutrons is to excite delayed and prompt gamma rays from elements in materials. This forms the basis of neutron activation analysis (NAA) and prompt gamma neutron activation analysis (PGNAA). NAA is most often used to analyze small samples of materials in a nuclear reactor whilst PGNAA is most often used to analyze subterranean rocks around bore holes and industrial bulk materials on conveyor belts.
Another use of neutron emitters is the detection of light nuclei, in particular the hydrogen found in water molecules. When a fast neutron collides with a light nucleus, it loses a large fraction of its energy. By measuring the rate at which slow neutrons return to the probe after reflecting off of hydrogen nuclei, a neutron probe may determine the water content in soil.
Medical therapies
Because neutron radiation is both penetrating and ionizing, it can be exploited for medical treatments. Neutron radiation can have the unfortunate side-effect of leaving the affected area radioactive, however. Neutron tomography is therefore not a viable medical application.Fast neutron therapy utilizes high energy neutrons typically greater than 20 MeV to treat cancer. Radiation therapy of cancers is based upon the biological response of cells to ionizing radiation. If radiation is delivered in small sessions to damage cancerous areas, normal tissue will have time to repair itself, while tumor cells often cannot.[96] Neutron radiation can deliver energy to a cancerous region at a rate an order of magnitude larger than gamma radiation[97]
Beams of low energy neutrons are used in boron capture therapy to treat cancer. In boron capture therapy, the patient is given a drug that contains boron and that preferentially accumulates in the tumor to be targeted. The tumor is then bombarded with very low energy neutrons (although often higher than thermal energy) which are captured by the boron-10 isotope in the boron, which produces an excited state of boron-11 that then decays to produce lithium-7 and an alpha particle that have sufficient energy to kill the malignant cell, but insufficient range to damage nearby cells. For such a therapy to be applied to the treatment of cancer, a neutron source having an intensity of the order of billion (109) neutrons per second per cm2 is preferred. Such fluxes require a research nuclear reactor.
Protection
Exposure to free neutrons can be hazardous, since the interaction of neutrons with molecules in the body can cause disruption to molecules and atoms, and can also cause reactions that give rise to other forms of radiation (such as protons). The normal precautions of radiation protection apply: Avoid exposure, stay as far from the source as possible, and keep exposure time to a minimum. Some particular thought must be given to how to protect from neutron exposure, however. For other types of radiation, e.g. alpha particles, beta particles, or gamma rays, material of a high atomic number and with high density make for good shielding; frequently, lead is used. However, this approach will not work with neutrons, since the absorption of neutrons does not increase straightforwardly with atomic number, as it does with alpha, beta, and gamma radiation. Instead one needs to look at the particular interactions neutrons have with matter (see the section on detection above). For example, hydrogen-rich materials are often used to shield against neutrons, since ordinary hydrogen both scatters and slows neutrons. This often means that simple concrete blocks or even paraffin-loaded plastic blocks afford better protection from neutrons than do far more dense materials. After slowing, neutrons may then be absorbed with an isotope that has high affinity for slow neutrons without causing secondary capture radiation, such as lithium-6.Hydrogen-rich ordinary water affects neutron absorption in nuclear fission reactors: Usually, neutrons are so strongly absorbed by normal water that fuel enrichment with fissionable isotope is required. The deuterium in heavy water has a very much lower absorption affinity for neutrons than does protium (normal light hydrogen). Deuterium is, therefore, used in CANDU-type reactors, in order to slow (moderate) neutron velocity, to increase the probability of nuclear fission compared to neutron capture.
Neutron temperature
Thermal neutrons
A thermal neutron is a free neutron that is Boltzmann distributed with kT = 0.0253 eV (4.0×10−21 J) at room temperature. This gives characteristic (not average, or median) speed of 2.2 km/s. The name 'thermal' comes from their energy being that of the room temperature gas or material they are permeating. (see kinetic theory for energies and speeds of molecules). After a number of collisions (often in the range of 10–20) with nuclei, neutrons arrive at this energy level, provided that they are not absorbed.In many substances, thermal neutron reactions show a much larger effective cross-section than reactions involving faster neutrons, and thermal neutrons can therefore be absorbed more readily (i.e., with higher probability) by any atomic nuclei that they collide with, creating a heavier — and often unstable — isotope of the chemical element as a result.
Most fission reactors use a neutron moderator to slow down, or thermalize the neutrons that are emitted by nuclear fission so that they are more easily captured, causing further fission. Others, called fast breeder reactors, use fission energy neutrons directly.
Cold neutrons
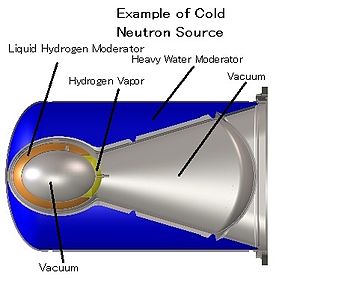
Cold neutrons are thermal neutrons that have been equilibrated in a very cold substance such as liquid deuterium. Such a cold source is placed in the moderator of a research reactor or spallation source.Cold neutrons are particularly valuable for neutron scattering experiments.[citation needed]
Ultracold neutrons
Ultracold neutrons are produced by inelastically scattering cold neutrons in substances with a temperature of a few kelvins, such as solid deuterium or superfluid helium. An alternative production method is the mechanical deceleration of cold neutrons.Fission energy neutrons
A fast neutron is a free neutron with a kinetic energy level close to 1 MeV (1.6×10−13 J), hence a speed of ~14000 km/s (~ 5% of the speed of light). They are named fission energy or fast neutrons to distinguish them from lower-energy thermal neutrons, and high-energy neutrons produced in cosmic showers or accelerators. Fast neutrons are produced by nuclear processes such as nuclear fission.
Neutrons produced in fission, as noted above, have a Maxwell–Boltzmann distribution of kinetic energies from 0 to ~14 MeV, a mean energy of 2 MeV (for U-235 fission neutrons), and a mode of only 0.75 MeV, which means that more than half of them do not qualify as fast (and thus have almost no chance of initiating fission in fertile materials, such as U-238 and Th-232).Fast neutrons can be made into thermal neutrons via a process called moderation. This is done with a neutron moderator. In reactors, typically heavy water, light water, or graphite are used to moderate neutrons.
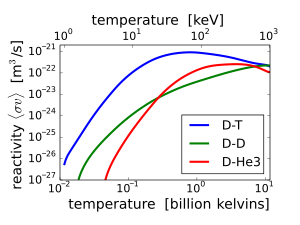
D–T (deuterium–tritium) fusion is the fusion reaction that produces the most energetic neutrons, with 14.1 MeV of kinetic energy and traveling at 17% of the speed of light. D–T fusion is also the easiest fusion reaction to ignite, reaching near-peak rates even when the deuterium and tritium nuclei have only a thousandth as much kinetic energy as the 14.1 MeV that will be produced.
14.1 MeV neutrons have about 10 times as much energy as fission neutrons, and are very effective at fissioning even non-fissile heavy nuclei, and these high-energy fissions produce more neutrons on average than fissions by lower-energy neutrons. This makes D–T fusion neutron sources such as proposed tokamak power reactors useful for transmutation of transuranic waste. 14.1 MeV neutrons can also produce neutrons by knocking them loose from nuclei.
On the other hand, these very high energy neutrons are less likely to simply be captured without causing fission or spallation. For these reasons, nuclear weapon design extensively utilizes D–T fusion 14.1 MeV neutrons to cause more fission. Fusion neutrons are able to cause fission in ordinarily non-fissile materials, such as depleted uranium (uranium-238), and these materials have been used in the jackets of thermonuclear weapons. Fusion neutrons also can cause fission in substances that are unsuitable or difficult to make into primary fission bombs, such as reactor grade plutonium. This physical fact thus causes ordinary non-weapons grade materials to become of concern in certain nuclear proliferation discussions and treaties.
Other fusion reactions produce much less energetic neutrons. D–D fusion produces a 2.45 MeV neutron and helium-3 half of the time, and produces tritium and a proton but no neutron the other half of the time. D–3He fusion produces no neutron.
A fission energy neutron that has slowed down but not yet reached thermal energies is called an epithermal neutron.
Cross sections for both capture and fission reactions often have multiple resonance peaks at specific energies in the epithermal energy range. These are of less significance in a fast neutron reactor, where most neutrons are absorbed before slowing down to this range, or in a well-moderated thermal reactor, where epithermal neutrons interact mostly with moderator nuclei, not with either fissile or fertile actinide nuclides. However, in a partially moderated reactor with more interactions of epithermal neutrons with heavy metal nuclei, there are greater possibilities for transient changes in reactivity that might make reactor control more difficult.
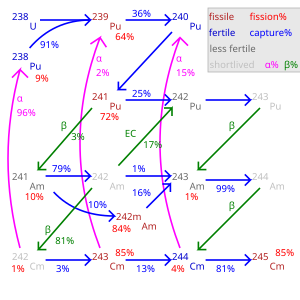
Ratios of capture reactions to fission reactions are also worse (more captures without fission) in most nuclear fuels such as plutonium-239, making epithermal-spectrum reactors using these fuels less desirable, as captures not only waste the one neutron captured but also usually result in a nuclide that is not fissile with thermal or epithermal neutrons, though still fissionable with fast neutrons. The exception is uranium-233 of the thorium cycle, which has good capture-fission ratios at all neutron energies.
Fusion neutrons
14.1 MeV neutrons have about 10 times as much energy as fission neutrons, and are very effective at fissioning even non-fissile heavy nuclei, and these high-energy fissions produce more neutrons on average than fissions by lower-energy neutrons. This makes D–T fusion neutron sources such as proposed tokamak power reactors useful for transmutation of transuranic waste. 14.1 MeV neutrons can also produce neutrons by knocking them loose from nuclei.
On the other hand, these very high energy neutrons are less likely to simply be captured without causing fission or spallation. For these reasons, nuclear weapon design extensively utilizes D–T fusion 14.1 MeV neutrons to cause more fission. Fusion neutrons are able to cause fission in ordinarily non-fissile materials, such as depleted uranium (uranium-238), and these materials have been used in the jackets of thermonuclear weapons. Fusion neutrons also can cause fission in substances that are unsuitable or difficult to make into primary fission bombs, such as reactor grade plutonium. This physical fact thus causes ordinary non-weapons grade materials to become of concern in certain nuclear proliferation discussions and treaties.
Other fusion reactions produce much less energetic neutrons. D–D fusion produces a 2.45 MeV neutron and helium-3 half of the time, and produces tritium and a proton but no neutron the other half of the time. D–3He fusion produces no neutron.
Intermediate-energy neutrons
A fission energy neutron that has slowed down but not yet reached thermal energies is called an epithermal neutron.
Cross sections for both capture and fission reactions often have multiple resonance peaks at specific energies in the epithermal energy range. These are of less significance in a fast neutron reactor, where most neutrons are absorbed before slowing down to this range, or in a well-moderated thermal reactor, where epithermal neutrons interact mostly with moderator nuclei, not with either fissile or fertile actinide nuclides. However, in a partially moderated reactor with more interactions of epithermal neutrons with heavy metal nuclei, there are greater possibilities for transient changes in reactivity that might make reactor control more difficult.
Ratios of capture reactions to fission reactions are also worse (more captures without fission) in most nuclear fuels such as plutonium-239, making epithermal-spectrum reactors using these fuels less desirable, as captures not only waste the one neutron captured but also usually result in a nuclide that is not fissile with thermal or epithermal neutrons, though still fissionable with fast neutrons. The exception is uranium-233 of the thorium cycle, which has good capture-fission ratios at all neutron energies.