Original link: http://www.desmoinesregister.com/story/money/agriculture/2015/03/20/glyphosate-carcinogen/25113437/
LONDON –One of the world's most popular weed-killers — and the most widely used kind in the United States — has been labeled a probable carcinogen by the International Agency for Research on Cancer.
The decision was made by the France-based cancer research arm of the World Health Organization, which considered the status of five insect and weed killers including glyphosate, which is used globally in industrial farming.
Monsanto, whose Roundup products contain glyphosate, said the determination was contrary to the facts.
"We don't know how IARC could reach a conclusion that is such a dramatic departure from the conclusion reached by all regulatory agencies around the globe," Monsanto's Phil Miller, global head of regulatory and government affairs, said in a statement.
The U.S. Environmental Protection Agency, which makes its own determinations, said it would consider the French agency's evaluation.
The French agency has four levels of risks for possible cancer-causing agents: known carcinogens, probable or possible carcinogens, not classifiable and probably not carcinogenic. Glyphosate now falls in the second level of concern.
The new classification is aimed mainly at industrial use of glyphosate. Its use by home gardeners is not considered a risk. Glyphosate is in the same category of risk as things like anabolic steroids and shift work. The decision was published online Thursday in the journal Lancet Oncology.
According to the French agency, glyphosate is used in more than 750 different herbicide products and its use has been detected in the air during spraying, in water and in food. Experts said there was "limited evidence" in humans that the herbicide can cause non-Hodgkin's lymphoma and there is convincing evidence that glyphosate can also cause other forms of cancer in rats and mice. The agency's panel said glyphosate has been found in the blood and urine of agricultural workers, showing the chemical has been absorbed by the body.
Monsanto and other producers of glyphosate-containing herbicides strongly disagreed with the decision. "All labeled uses of glyphosate are safe for human health," said Miller, the Monsanto executive.
The EPA's 2012 assessment of glyphosate concluded that it met the statutory safety standards and that the chemical could "continue to be used without unreasonable risks to people or the environment."
The French agency's experts said the cancer risks of the weed killer were mostly from occupational exposure.
"I don't think home use is the issue," said Kate Guyton of the International Agency for Research on Cancer. "It's agricultural use that will have the biggest impact. For the moment, it's just something for people to be conscious of."
A Medley of Potpourri is just what it says; various thoughts, opinions, ruminations, and contemplations on a variety of subjects.
Search This Blog
Thursday, April 9, 2015
Carcinogen
From Wikipedia, the free encyclopedia

The international pictogram for chemicals that are sensitising, mutagenic, carcinogenic or toxic to reproduction.
A carcinogen is any substance, radionuclide, or radiation that is an agent directly involved in causing cancer. This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substances are considered carcinogens, but their carcinogenic activity is attributed to the radiation, for example gamma rays and alpha particles, which they emit. Common examples of non-radioactive carcinogens are inhaled asbestos, certain dioxins, and tobacco smoke. Although the public generally associates carcinogenicity with synthetic chemicals, it is equally likely to arise in both natural and synthetic substances.[1] Carcinogens are not necessarily immediately toxic, thus their effect can be insidious.
Cancer is any disease in which normal cells are damaged and do not undergo programmed cell death as fast as they divide via mitosis. Carcinogens may increase the risk of cancer by altering cellular metabolism or damaging DNA directly in cells, which interferes with biological processes, and induces the uncontrolled, malignant division, ultimately leading to the formation of tumors. Usually, severe DNA damage leads to apoptosis, but if the programmed cell death pathway is damaged, then the cell cannot prevent itself from becoming a cancer cell.
There are many natural carcinogens. Aflatoxin B1, which is produced by the fungus Aspergillus flavus growing on stored grains, nuts and peanut butter, is an example of a potent, naturally occurring microbial carcinogen. Certain viruses such as hepatitis B and human papilloma virus have been found to cause cancer in humans. The first one shown to cause cancer in animals is Rous sarcoma virus, discovered in 1910 by Peyton Rous. Other infectious organisms which cause cancer in humans include some bacteria (e.g. Helicobacter pylori [2][3]) and helminths (e.g. Opisthorchis viverrini [4] and Clonorchis sinensis [5]).
Dioxins and dioxin-like compounds, benzene, kepone, EDB, and asbestos have all been classified as carcinogenic.[6] As far back as the 1930s, industrial smoke and tobacco smoke were identified as sources of dozens of carcinogens, including benzo[a]pyrene, tobacco-specific nitrosamines such as nitrosonornicotine, and reactive aldehydes such as formaldehyde—which is also a hazard in embalming and making plastics. Vinyl chloride, from which PVC is manufactured, is a carcinogen and thus a hazard in PVC production.
Co-carcinogens are chemicals that do not necessarily cause cancer on their own, but promote the activity of other carcinogens in causing cancer.
After the carcinogen enters the body, the body makes an attempt to eliminate it through a process called biotransformation. The purpose of these reactions is to make the carcinogen more water-soluble so that it can be removed from the body. However, in some cases, these reactions can also convert a less toxic carcinogen into a more toxic carcinogen.
DNA is nucleophilic, therefore soluble carbon electrophiles are carcinogenic, because DNA attacks them. For example, some alkenes are toxicated by human enzymes to produce an electrophilic epoxide. DNA attacks the epoxide, and is bound permanently to it. This is the mechanism behind the carcinogenicity of benzo[a]pyrene in tobacco smoke, other aromatics, aflatoxin and mustard gas.
Radiation
CERCLA identifies all radionuclides as carcinogens, although the nature of the emitted radiation (alpha, beta, gamma, or neutron and the radioactive strength), its consequent capacity to cause ionization in tissues, and the magnitude of radiation exposure, determine the potential hazard. Carcinogenicity of radiation depends of the type of radiation, type of exposure, and penetration. For example, alpha radiation has low penetration and is not a hazard outside the body, but emitters are carcinogenic when inhaled or ingested.For example, Thorotrast, a (incidentally radioactive) suspension previously used as a contrast medium in x-ray diagnostics, is a potent human carcinogen known because of its retention within various organs and persistent emission of alpha particles.
Not all types of electromagnetic radiation are carcinogenic. Low-energy waves on the electromagnetic spectrum including radio waves, microwaves, infrared radiation and visible light are thought not to be, because they have insufficient energy to break chemical bonds. Evidence for carcinogenic effects of non-ionizing radiation is generally inconclusive, though there are some documented cases of radar technicians with prolonged high exposure experiencing significantly higher cancer incidence.[8] Higher-energy radiation, including ultraviolet radiation (present in sunlight), x-rays, and gamma radiation, generally is carcinogenic, if received in sufficient doses.
Low level ionizing radiation may induce irreparable DNA damage (leading to replicational and transcriptional errors needed for neoplasia or may trigger viral interactions) leading to pre-mature aging and cancer.[9][10][11]
Substances or foods irradiated with electrons or electromagnetic radiation (such as microwave, X-ray or gamma) are not carcinogenic.[citation needed] In contrast, non-electromagnetic neutron radiation produced inside nuclear reactors can produce secondary radiation through nuclear transmutation.
In prepared food
Cooking food at high temperatures, for example grilling or barbecuing meats, can lead to the formation of minute quantities of many potent carcinogens that are comparable to those found in cigarette smoke (i.e., benzo[a]pyrene).[12] Charring of food resembles coking and tobacco pyrolysis, and produces similar carcinogens.
There are several carcinogenic pyrolysis products, such as polynuclear aromatic hydrocarbons, which are converted by human enzymes into epoxides, which attach permanently to DNA. Pre-cooking meats in a microwave oven for 2–3 minutes before grilling shortens the time on the hot pan, and removes heterocyclic amine (HCA) precursors, which can help minimize the formation of these carcinogens.[13]
Reports from the Food Standards Agency have found that the known animal carcinogen acrylamide is generated in fried or overheated carbohydrate foods (such as french fries and potato chips).[14] Studies are underway at the FDA and European regulatory agencies to assess its potential risk to humans.
Nongenotoxins do not directly affect DNA but act in other ways to promote growth. These include hormones and some organic compounds.[15]
Dietary increases in total fat or saturated fat result in elevated DCA and LCA in feces and elevated exposure of the colon epithelium to these bile acids. When the bile acid DCA was added to the standard diet of wild-type mice invasive colon cancer was induced in 56% of the mice after 8 to 10 months.[38] Overall, the available evidence indicates that DCA and LCA are centrally important DNA-damaging carcinogens in colon cancer.
Reports from the Food Standards Agency have found that the known animal carcinogen acrylamide is generated in fried or overheated carbohydrate foods (such as french fries and potato chips).[14] Studies are underway at the FDA and European regulatory agencies to assess its potential risk to humans.
In cigarettes
Mechanisms of carcinogenicity
Carcinogens can be classified as genotoxic or nongenotoxic. Genotoxins cause irreversible genetic damage or mutations by binding to DNA. Genotoxins include chemical agents like N-nitroso-N-methylurea (NMU) or non-chemical agents such as ultraviolet light and ionizing radiation. Certain viruses can also act as carcinogens by interacting with DNA.Nongenotoxins do not directly affect DNA but act in other ways to promote growth. These include hormones and some organic compounds.[15]
Classification
International Agency for Research on Cancer
The International Agency for Research on Cancer (IARC) is an intergovernmental agency established in 1965, which forms part of the World Health Organization of the United Nations. It is based in Lyon, France. Since 1971 it has published a series of Monographs on the Evaluation of Carcinogenic Risks to Humans[16] that have been highly influential in the classification of possible carcinogens.- Group 1: the agent (mixture) is definitely carcinogenic to humans. The exposure circumstance entails exposures that are carcinogenic to humans.
- Group 2A: the agent (mixture) is probably carcinogenic to humans. The exposure circumstance entails exposures that are probably carcinogenic to humans.
- Group 2B: the agent (mixture) is possibly carcinogenic to humans. The exposure circumstance entails exposures that are possibly carcinogenic to humans.
- Group 3: the agent (mixture or exposure circumstance) is not classifiable as to its carcinogenicity to humans.
- Group 4: the agent (mixture) is probably not carcinogenic to humans.
Globally Harmonized System
The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) is a United Nations initiative to attempt to harmonize the different systems of assessing chemical risk which currently exist (as of March 2009) around the world. It classifies carcinogens into two categories, of which the first may be divided again into subcategories if so desired by the competent regulatory authority:- Category 1: known or presumed to have carcinogenic potential for humans
- Category 1A: the assessment is based primarily on human evidence
- Category 1B: the assessment is based primarily on animal evidence
- Category 2: suspected human carcinogens
U.S. National Toxicology Program
The National Toxicology Program of the U.S. Department of Health and Human Services is mandated to produce a biennial Report on Carcinogens.[17] As of June 2011, the latest edition was the 12th report (2011).[6] It classifies carcinogens into two groups:- Known to be a human carcinogen
- Reasonably anticipated to be a human carcinogen
American Conference of Governmental Industrial Hygienists
The American Conference of Governmental Industrial Hygienists (ACGIH) is a private organization best known for its publication of threshold limit values (TLVs) for occupational exposure and monographs on workplace chemical hazards. It assesses carcinogenicity as part of wider assessment of the occupational hazards of chemicals.- Group A1: Confirmed human carcinogen
- Group A2: Suspected human carcinogen
- Group A3: Confirmed animal carcinogen with unknown relevance to humans
- Group A4: Not classifiable as a human carcinogen
- Group A5: Not suspected as a human carcinogen
European Union
The European Union classification of carcinogens is contained in the Dangerous Substances Directive and the Dangerous Preparations Directive. It consists of three categories:- Category 1: Substances known to be carcinogenic to humans.
- Category 2: Substances which should be regarded as if they are carcinogenic to humans.
- Category 3: Substances which cause concern for humans, owing to possible carcinogenic effects but in respect of which the available information is not adequate for making a satisfactory assessment.
Safe Work Australia
Under a previous name, the NOHSC, in 1999 Safe Work Australia published the Approved Criteria for Classifying Hazardous Substances [NOHSC:1008(1999)].[18] Section 4.76 of this document outlines the criteria for classifying carcinogens as approved by the Australian government. This classification consists of three categories:- Category 1: Substances known to be carcinogenic to humans.
- Category 2: Substances that should be regarded as if they were carcinogenic to humans.
- Category 3: Substances that have possible carcinogenic effects in humans but about which there is insufficient information to make an assessment.
Procarcinogen
A procarcinogen is a precursor to a carcinogen. One example is nitrites when taken in by the diet. They are not carcinogenic themselves, but turn into nitrosamines in the body, which are carcinogenic.[19]Common carcinogens
Occupational carcinogens
Occupational carcinogens are agents that pose a risk of cancer in several specific work-locations:| Carcinogen | Associated cancer sites or types | Occupational uses or sources |
|---|---|---|
| Arsenic and its compounds |
|
|
| Asbestos |
|
Not in widespread use, but found in:
|
| Benzene |
|
|
| Beryllium and its compounds |
|
|
| Cadmium and its compounds[20] | ||
| Hexavalent chromium(VI) compounds |
|
|
| IC engine exhaust gas |
|
|
| Ethylene oxide |
|
|
| Nickel |
|
|
| Radon and its decay products |
|
|
| Vinyl chloride |
|
|
| Shift work that involves
circadian disruption[22] |
||
| Involuntary smoking (Passive smoking)[23] |
|
|
| Radium-226, Radium-224, Plutonium-238, Plutonium-239[24] and other alpha particle emitters with high atomic weight |
|
|
| Unless otherwise specified, ref is:[25] |
Others
- Gasoline (contains aromatics)
- Lead and its compounds
- Alkylating antineoplastic agents (e.g. mechlorethamine)
- Other alkylating agents (e.g. dimethyl sulfate)
- Ultraviolet radiation from the sun and UV lamps
- Alcohol (causing head and neck cancers)
- Other ionizing radiation (X-rays, gamma rays, etc.)
Major carcinogens implicated in the four most common cancers worldwide
In this section, the carcinogens implicated as the main causative agents of the four most common cancers worldwide are briefly described. These four cancers are lung, breast, colon, and stomach cancers. Together they account for about 41% of worldwide cancer incidence and 42% of cancer deaths (for more detailed information on the carcinogens implicated in these and other cancers, see references[26][27]).Lung cancer
Lung cancer is the most common cancer in the world, both in terms of cases (1.6 million cases; 12.7% of total cancer cases) and deaths (1.4 million deaths; 18.2% of total cancer deaths).[28] Lung cancer is largely caused by tobacco smoke. Risk estimates for lung cancer in the United States indicate that tobacco smoke is responsible for 90% of lung cancers. Other factors are implicated in lung cancer, and these factors can interact synergistically with smoking, so that total attributable risk adds up to more than 100%. These factors include occupational exposure to carcinogens (about 9-15%), radon (10%) and outdoor air pollution (1-2%).[29] Tobacco smoke is a complex mixture of more than 5,300 identified chemicals. The most important carcinogens in tobacco smoke have been determined by a “Margin of Exposure” approach.[30] Using this approach, the most important tumorigenic compounds in tobacco smoke were, in order of importance, acrolein, formaldehyde, acrylonitrile, 1,3-butadiene, cadmium, acetaldehyde, ethylene oxide and isoprene. Most of these compounds cause DNA damage by forming DNA adducts or by inducing other alterations in DNA.[27] DNA damages are subject to error-prone DNA repair or can cause replication errors. Such errors in repair or replication can result in mutations in tumor suppressor genes or oncogenes leading to cancer.Breast cancer
Breast cancer is the second most common cancer [(1.4 million cases, 10.9%), but ranks 5th as cause of death (458,000, 6.1%)].[28] Increased risk of breast cancer is associated with persistently elevated blood levels of estrogen.[31] Estrogen appears to contribute to breast carcinogenesis by three processes; (1) the metabolism of estrogen to genotoxic, mutagenic carcinogens, (2) the stimulation of tissue growth, and (3) the repression of phase II detoxification enzymes that metabolize ROS leading to increased oxidative DNA damage.[32][33][34] The major estrogen in humans, estradiol, can be metabolized to quinone derivatives that form adducts with DNA.[35] These derivatives can cause dupurination, the removal of bases from the phosphodiester backbone of DNA, followed by inaccurate repair or replication of the apurinic site leading to mutation and eventually cancer. This genotoxic mechanism may interact in synergy with estrogen receptor-mediated, persistent cell proliferation to ultimately cause breast cancer.[35] Genetic background, dietary practices and environmental factors also likely contribute to the incidence of DNA damage and breast cancer risk.Colon cancer
Colorectal cancer is the third most common cancer [1.2 million cases (9.4%), 608,000 deaths (8.0%)].[28] Tobacco smoke may be responsible for up to 20% of colorectal cancers in the United States.[36] In addition, substantial evidence implicates bile acids as an important factor in colon cancer. Twelve studies (summarized in Bernstein et al.[37]) indicate that the bile acids deoxycholic acid (DCA) and/or lithocholic acid (LCA) induce production of DNA damaging reactive oxygen species and/or reactive nitrogen species in human or animal colon cells. Furthermore 14 studies showed that DCA and LCA induce DNA damage in colon cells. Also 27 studies reported that bile acids cause programmed cell death (apoptosis). Increased apoptosis can result in selective survival of cells that are resistant to induction of apoptosis.[37] Colon cells with reduced ability to undergo apoptosis in response to DNA damage would tend to accumulate mutations, and such cells may give rise to colon cancer.[37] Epidemiologic studies have found that fecal bile acid concentrations are increased in populations with a high incidence of colon cancer.Dietary increases in total fat or saturated fat result in elevated DCA and LCA in feces and elevated exposure of the colon epithelium to these bile acids. When the bile acid DCA was added to the standard diet of wild-type mice invasive colon cancer was induced in 56% of the mice after 8 to 10 months.[38] Overall, the available evidence indicates that DCA and LCA are centrally important DNA-damaging carcinogens in colon cancer.
Stomach cancer
Stomach cancer is the fourth most common cancer [990,000 cases (7.8%), 738,000 deaths (9.7%)].[28] Helicobacter pylori infection is the main causative factor in stomach cancer. Chronic gastritis (inflammation) caused by H. pylori is often long-standing if not treated. Infection of gastric epithelial cells with H. pylori results in increased production of reactive oxygen species (ROS).[39][40] ROS cause oxidative DNA damage including the major base alteration 8-hydroxydeoxyguanosine (8-OHdG). 8-OHdG resulting from ROS is increased in chronic gastritis. The altered DNA base can cause errors during DNA replication that have mutagenic and carcinogenic potential. Thus H. pylori-induced ROS appear to be the major carcinogens in stomach cancer because they cause oxidative DNA damage leading to carcinogenic mutations.Experimental cancer treatment
From Wikipedia, the free encyclopedia
The entries listed below vary between theoretical therapies to unproven controversial therapies. Many of these treatments are alleged to help against only specific forms of cancer. It is not a list of treatments widely available at hospitals.
Studying treatments for cancer
The twin goals of research are to determine whether the treatment actually works (called efficacy) and whether it is sufficiently safe. Regulatory processes attempt to balance the potential benefits with the potential harms, so that people given the treatment are more likely to benefit from it than to be harmed by it.Medical research for cancer begins much like research for any disease. In organized studies of new treatments for cancer, the pre-clinical development of drugs, devices, and techniques begins in laboratories, either with isolated cells or in small animals, most commonly rats or mice. In other cases, the proposed treatment for cancer is already in use for some other medical condition, in which case more is known about its safety and potential efficacy.
Clinical trials are the study of treatments in humans. The first-in-human tests of a potential treatment are called Phase I studies. Early clinical trials typically enroll a very small number of patients, and the purpose is to identify major safety issues and the maximum tolerated dose, which is the highest dose that does not produce serious or fatal adverse effects. The dose given in these trials may be far too small to produce any useful effect. In most research, these early trials may involve healthy people, but cancer studies normally enroll only people with relatively severe forms of the disease in this stage of testing. On average, 95% of the participants in these early trials receive no benefit, but all are exposed to the risk of adverse effects.[1] Most participants show signs of optimism bias (the irrational belief that they will beat the odds).
Later studies, called Phase II and Phase III studies, enroll more people, and the goal is to determine whether the treatment actually works. Phase III studies are frequently randomized controlled trials, with the experimental treatment being compared to the current best available treatment rather than to a placebo. In some cases, the Phase III trial provides the best available treatment to all participants, in addition to some of the patients receiving the experimental treatment.
Bacterial treatments
Chemotherapeutic drugs have a hard time penetrating tumors to kill them at their core because these cells may lack a good blood supply. Researchers have been using anaerobic bacteria, such as Clostridium novyi, to consume the interior of oxygen-poor tumours. These should then die when they come in contact with the tumour's oxygenated sides, meaning they would be harmless to the rest of the body. A major problem has been that bacteria do not consume all parts of the malignant tissue. However, combining the therapy with chemotheraputic treatments can help to solve this problem.Another strategy is to use anaerobic bacteria that have been transformed with an enzyme that can convert a non-toxic prodrug into a toxic drug. With the proliferation of the bacteria in the necrotic and hypoxic areas of the tumour, the enzyme is expressed solely in the tumour. Thus, a systemically applied prodrug is metabolised to the toxic drug only in the tumour. This has been demonstrated to be effective with the nonpathogenic anaerobe Clostridium sporogenes.[2]
Drug therapies
HAMLET (human alpha-lactalbumin made lethal to tumor cells)
HAMLET (human alpha-lactalbumin made lethal to tumor cells) is a molecular complex derived from human breast milk that kills tumor cells by a process resembling programmed cell death (apoptosis). It has been tested in humans with skin papillomas and bladder cancer.[3]Dichloroacetate
Dichloroacetate (DCA) has been found to shrink tumors in vivo in rats, and has a plausible scientific mechanism: DCA appears to reactivate suppressed mitochondria in some types of oxygen-starved tumor cells, and thus promotes apoptosis.[4] Because it was tested for other conditions, DCA is known to be relatively safe, available, and inexpensive, and it can be taken by mouth as a pill, which is convenient. Five patients with brain cancer have been treated with DCA in a clinical trial, and the authors say that the lives of four were 'probably' extended.[5][6] However, without a large controlled trial it is impossible to say whether the drug is truly effective against cancer.[7][8]Quercetin
Quercetin is a principal flavonoid compound and an excellent free-radical-scavenging antioxidant that promotes apoptosis. In vitro it shows some antitumor activity in oral cancer and leukemia.[9][10][11] Cultured skin and prostate cancer cells showed significant mortality (compared to nonmalignant cells) when treated with a combination of quercetin and ultrasound[12] Note that ultrasound also promotes topical absorption by up to 1,000 times, making the use of topical quercetin and ultrasound wands an interesting proposition.[13]High dietary intake of fruits and vegetables is associated with reduction in cancer, and some scientists, such as Gian Luigi Russo at the Institute of Food Sciences in Italy, suspect quercetin may be partly responsible.[14][15] Research shows that quercetin influences cellular mechanisms in vitro and in animal studies.[16] According to the American Cancer society, "there is no reliable clinical evidence that quercetin can prevent or treat cancer in humans".[17]
Insulin potentiation therapy
Insulin potentiation therapy is practice of injecting insulin, usually alongside conventional cancer drugs, in the belief that this improves the overall effect of the treatment. Quackwatch state: "Insulin Potentiation Therapy (IPT) is one of several unproven, dangerous treatments that is promoted by a small group of practitioners without trustworthy evidence that it works." [18]Drugs that restore p53 activity
Several drug therapies are being developed based on p53, the tumour suppressor gene that protects the cell in response to damage and stress. It is analogous to deciding what to do with a damaged car: p53 brings everything to a halt, and then decides whether to fix the cell or, if the cell is beyond repair, to destroy the cell. This protective function of p53 is disabled in most cancer cells, allowing them to multiply without check. Restoration of p53 activity in tumours (where possible) has been shown to inhibit tumour growth and can even shrink the tumour.[19][20][21]As p53 protein levels are usually kept low, one could block its degradation and allow large amounts of p53 to accumulate, thus stimulating p53 activity and its antitumour effects. Drugs that utilize this mechanism include nutlin and MI-219, which are both in phase I clinical trials.[22] There are also other drugs that are still in the preclinical stage of testing, such as RITA[23] and MITA.[24]
BI811283
BI811283 is a small molecule inhibitor of the Aurora B kinase protein being developed by Boehringer Ingelheim for use as an anti-cancer agent. BI 811283 is currently in the early stages of clinical development and is undergoing first-in-human trials in patients with solid tumors and Acute Myeloid Leukaemia.[25]Gene therapy
Introduction of tumor suppressor genes into rapidly dividing cells has been thought to slow down or arrest tumor growth. Adenoviruses are a commonly utilized vector for this purpose. Much research has focused on the use of adenoviruses that cannot reproduce, or reproduce only to a limited extent, within the patient to ensure safety via the avoidance of cytolytic destruction of noncancerous cells infected with the vector. However, new studies focus on adenoviruses that can be permitted to reproduce, and destroy cancerous cells in the process, since the adenoviruses' ability to infect normal cells is substantially impaired, potentially resulting in a far more effective treatment.[26][27]
Another use of gene therapy is the introduction of enzymes into these cells that make them susceptible to particular chemotherapy agents; studies with introducing thymidine kinase in gliomas, making them susceptible to aciclovir, are in their experimental stage.
Epigenetic Options
Epigenetics is the study of heritable changes in gene activity that are not caused by changes in the DNA sequence, often a result of environmental or dietary damage to the histone receptors within the cell. Current research has shown that epigenetic pharmaceuticals could be a putative replacement or adjuvant therapy for currently accepted treatment methods such as radiation and chemotherapy, or could enhance the effects of these current treatments.[28]
It has been shown that the epigenetic control of the proto-onco regions and the tumor suppressor sequences by conformational changes in histones directly affects the formation and progression of cancer.[29] Epigenetics also has the factor of reversibility, a characteristic that other cancer treatments do not offer.[30]Some investigators, like Randy Jirtle, PhD, of Duke University Medical Center, think epigenetics may ultimately turn out to have a greater role in disease than genetics.[31]
A number of research groups have experimented with the use of telomerase inhibitors in animal models, and as of 2005 and 2006 phase I and II human clinical trials are underway. Geron Corporation is currently conducting two clinical trials involving telomerase inhibitors. One uses a vaccine (GRNVAC1) and the other uses a lipidated oligonucleotide(GRN163L).
More prolonged moderate heating to temperatures just a few degrees above normal (39,5°C) can cause more subtle changes. A mild heat treatment combined with other stresses can cause cell death by apoptosis. There are many biochemical consequences to the heat shock response within the cell, including slowed cell division and increased sensitivity to ionizing radiation therapy. The purpose of overheating the tumor cells is to create a lack of oxygen so that the heated cells become overacidified, which leads to a lack of nutrients in the tumor. This in turn disrupts the metabolism of the cells so that cell death (apoptosis) can set in. In certain cases chemotherapy or radiation that has previously not had any effect can be made effective. Hyperthermia alters the cell walls by means of so-called heat shock proteins. The cancer cells then react very much more effectively to the cytostatics and radiation. If hyperthermia is used conscientiously it has no serious side effects.[33]
There are many techniques by which heat may be delivered. Some of the most common involve the use of focused ultrasound (FUS or HIFU), microwave heating, induction heating, magnetic hyperthermia, and direct application of heat through the use of heated saline pumped through catheters. Experiments with carbon nanotubes that selectively bind to cancer cells have been performed. Lasers are then used that pass harmlessly through the body, but heat the nanotubes, causing the death of the cancer cells. Similar results have also been achieved with other types of nanoparticles, including gold-coated nanoshells and nanorods that exhibit certain degrees of 'tunability' of the absorption properties of the nanoparticles to the wavelength of light for irradiation. The success of this approach to cancer treatment rests on the existence of an 'optical window' in which biological tissue (i.e., healthy cells) are completely transparent at the wavelength of the laser light, while nanoparticles are highly absorbing at the same wavelength. Such a 'window' exists in the so-called near-infrared region of the electromagnetic spectrum. In this way, the laser light can pass through the system without harming healthy tissue, and only diseased cells, where the nanoparticles reside, get hot and are killed.
Magnetic hyperthermia makes use of magnetic nanoparticles, which can be injected into tumours and then generate heat when subjected to an alternating magnetic field.[34]
One of the challenges in thermal therapy is delivering the appropriate amount of heat to the correct part of the patient's body. A great deal of current research focuses on precisely positioning heat delivery devices (catheters, microwave, and ultrasound applicators, etc.) using ultrasound or magnetic resonance imaging, as well as of developing new types of nanoparticles that make them particularly efficient absorbers while offering little or no concerns about toxicity to the circulation system. Clinicians also hope to use advanced imaging techniques to monitor heat treatments in real time—heat-induced changes in tissue are sometimes perceptible using these imaging instruments.
Another method that is entirely non-invasive referred to as Tumor Treating Fields has already reached clinical trial stage in many countries. The concept applies an electric field through a tumour region using electrodes external to the body. Successful trials have shown the process effectiveness to be greater than chemotherapy and there are no side-effects and only negligible time spent away from normal daily activities.[38][39] This treatment is still in very early development stages for many types of cancer.
High-intensity focused ultrasound (HIFU) is still in investigatory phases in many places around the world. In China it has CFDA approval and over 180 treatment centres have been established in China, Hong Kong, and Korea. HIFU has been successfully used to treat cancer to destroy tumours of the bone, brain, breast, liver, pancreas, rectum, kidney, testes, and prostate. Several thousand patients have been treated with various types of tumours. HIFU has CE approval for palliative care for bone metastasis. Experimentally, palliative care has been provided for cases of advanced pancreatic cancer.
Most complementary and alternative medicines for cancer have not been rigorously studied or tested. Some alternative treatments that have been proven ineffective continue to be marketed and promoted.[44]
Telomerase therapy
Because most malignant cells rely on the activity of the protein telomerase for their immortality, it has been proposed that a drug that inactivates telomerase might be effective against a broad spectrum of malignancies. At the same time, most healthy tissues in the body express little if any telomerase, and would function normally in its absence. Currently, Inositol hexaphosphate, which is available over-the-counter, is undergoing testing in cancer research due to its telomerase-inhibiting abilities.[32]A number of research groups have experimented with the use of telomerase inhibitors in animal models, and as of 2005 and 2006 phase I and II human clinical trials are underway. Geron Corporation is currently conducting two clinical trials involving telomerase inhibitors. One uses a vaccine (GRNVAC1) and the other uses a lipidated oligonucleotide(GRN163L).
Radiation therapies
Photodynamic therapy
Photodynamic therapy (PDT) is generally a non-invasive treatment using a combination of light and a photosensitive drug, such as 5-ALA, Foscan, Metvix, Tookad, WST09, WST11, Photofrin, or Visudyne. The drug is triggered by light of a specific wavelength.Hyperthermia therapy
Localized and whole-body application of heat has been proposed as a technique for the treatment of malignant tumours. Intense heating will cause denaturation and coagulation of cellular proteins, rapidly killing cells within a tumour.More prolonged moderate heating to temperatures just a few degrees above normal (39,5°C) can cause more subtle changes. A mild heat treatment combined with other stresses can cause cell death by apoptosis. There are many biochemical consequences to the heat shock response within the cell, including slowed cell division and increased sensitivity to ionizing radiation therapy. The purpose of overheating the tumor cells is to create a lack of oxygen so that the heated cells become overacidified, which leads to a lack of nutrients in the tumor. This in turn disrupts the metabolism of the cells so that cell death (apoptosis) can set in. In certain cases chemotherapy or radiation that has previously not had any effect can be made effective. Hyperthermia alters the cell walls by means of so-called heat shock proteins. The cancer cells then react very much more effectively to the cytostatics and radiation. If hyperthermia is used conscientiously it has no serious side effects.[33]
There are many techniques by which heat may be delivered. Some of the most common involve the use of focused ultrasound (FUS or HIFU), microwave heating, induction heating, magnetic hyperthermia, and direct application of heat through the use of heated saline pumped through catheters. Experiments with carbon nanotubes that selectively bind to cancer cells have been performed. Lasers are then used that pass harmlessly through the body, but heat the nanotubes, causing the death of the cancer cells. Similar results have also been achieved with other types of nanoparticles, including gold-coated nanoshells and nanorods that exhibit certain degrees of 'tunability' of the absorption properties of the nanoparticles to the wavelength of light for irradiation. The success of this approach to cancer treatment rests on the existence of an 'optical window' in which biological tissue (i.e., healthy cells) are completely transparent at the wavelength of the laser light, while nanoparticles are highly absorbing at the same wavelength. Such a 'window' exists in the so-called near-infrared region of the electromagnetic spectrum. In this way, the laser light can pass through the system without harming healthy tissue, and only diseased cells, where the nanoparticles reside, get hot and are killed.
Magnetic hyperthermia makes use of magnetic nanoparticles, which can be injected into tumours and then generate heat when subjected to an alternating magnetic field.[34]
One of the challenges in thermal therapy is delivering the appropriate amount of heat to the correct part of the patient's body. A great deal of current research focuses on precisely positioning heat delivery devices (catheters, microwave, and ultrasound applicators, etc.) using ultrasound or magnetic resonance imaging, as well as of developing new types of nanoparticles that make them particularly efficient absorbers while offering little or no concerns about toxicity to the circulation system. Clinicians also hope to use advanced imaging techniques to monitor heat treatments in real time—heat-induced changes in tissue are sometimes perceptible using these imaging instruments.
Non-invasive cancer treatment
This preclinical treatment involves using radio waves to heat up tiny metals that are implanted in cancerous tissue. Gold nanoparticles or carbon nanotubes are the most likely candidate. Promising preclinical trials have been conducted,[35][36] although clinical trials may not be held for another few years.[37]Another method that is entirely non-invasive referred to as Tumor Treating Fields has already reached clinical trial stage in many countries. The concept applies an electric field through a tumour region using electrodes external to the body. Successful trials have shown the process effectiveness to be greater than chemotherapy and there are no side-effects and only negligible time spent away from normal daily activities.[38][39] This treatment is still in very early development stages for many types of cancer.
High-intensity focused ultrasound (HIFU) is still in investigatory phases in many places around the world. In China it has CFDA approval and over 180 treatment centres have been established in China, Hong Kong, and Korea. HIFU has been successfully used to treat cancer to destroy tumours of the bone, brain, breast, liver, pancreas, rectum, kidney, testes, and prostate. Several thousand patients have been treated with various types of tumours. HIFU has CE approval for palliative care for bone metastasis. Experimentally, palliative care has been provided for cases of advanced pancreatic cancer.
Electromagnetic treatments
Tumor Treating Fields is a novel FDA-approved cancer treatment therapy that uses alternating electric field to disturb the rapid cell division exhibited by cancer cells.[40]Complementary and alternative treatments
Complementary and alternative medicine (CAM) treatments are the diverse group of medical and healthcare systems, practices, and products that are not part of conventional medicine and have not been proven to be effective.[41] Complementary medicine usually refers to methods and substances used along with conventional medicine, while alternative medicine refers to compounds used instead of conventional medicine.[42] CAM use is common among people with cancer.[43]Most complementary and alternative medicines for cancer have not been rigorously studied or tested. Some alternative treatments that have been proven ineffective continue to be marketed and promoted.[44]
Oncology
From Wikipedia, the free encyclopedia
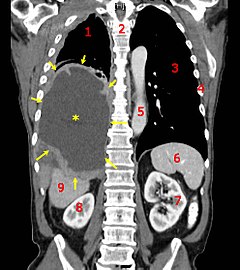
A coronal CT scan showing a malignant mesothelioma, indicated by the asterisk and the arrows
|
|
| Focus | Cancerous tumor |
|---|---|
| Subdivisions | Medical oncology, radiation oncology, surgical oncology |
| Significant tests | Tumor markers, TNM staging, CT scans, MRI |
| Specialist | Oncologist |
Oncology is a branch of medicine that deals with tumors. A medical professional who practices oncology is an oncologist. The name's etymological origin is the Greek word ὄγκος (ónkos), meaning "tumor", "volume" or "mass".[1]
Oncology is concerned with:
- The diagnosis of any cancer in a person (pathology)
- Therapy (e.g. surgery, chemotherapy, radiotherapy and other modalities)
- Follow-up of cancer patients after successful treatment
- Palliative care of patients with terminal malignancies
- Ethical questions surrounding cancer care
- Screening efforts:
- of populations, or
- of the relatives of patients (in types of cancer that are thought to have a hereditary basis, such as breast cancer)
Diagnosis
Medical histories remain an important screening tool: the character of the complaints and nonspecific symptoms (such as fatigue, weight loss, unexplained anemia, fever of unknown origin, paraneoplastic phenomena and other signs) may warrant further investigation for malignancy. Occasionally, a physical examination may find the location of a malignancy.Diagnostic methods include:
- Biopsy or Resection; these are methods by which suspicious neoplastic growths can be removed in part or in whole, and evaluated by a pathologist to determine malignancy. This is currently the gold standard for the diagnosis of cancer and is crucial in guiding the next step in management (active surveillance, surgery, radiation therapy, chemotherapy or a combination of these)
- Endoscopy, either upper or lower gastrointestinal, cystoscopy, bronchoscopy, or nasendoscopy; to localise areas suspicious for malignancy and biopsy when necessary.
- X-rays, CT scanning, MRI scanning, ultrasound and other radiological techniques to localise and guide biopsy.
- Scintigraphy, Single Photon Emission Computed Tomography (SPECT), Positron emission tomography (PET) and other methods of nuclear medicine to identify areas suspicious for malignancy.
- Blood tests, including tumor markers, which can increase the suspicion of certain types of cancers.
Currently, a tissue diagnosis (from a biopsy) by a pathologist is essential for the proper classification of cancer and to guide the next step of treatment. On extremely rare instances when this is not possible, "empirical therapy" (without an exact diagnosis) may be considered, based on the available evidence (e.g. history, x-rays and scans.)
On very rare occasions, a metastatic lump or pathological lymph node is found (typically in the neck) for which a primary tumor cannot be found. However, immunohistochemical markers often give a strong indication of the primary malignancy. This situation is referred to as "malignacy of unknown primary", and again, treatment is empirical based on past experience of the most likely origin.[2]
Therapy
Depending upon the cancer identified, followup and palliative care will be administered at that time. Certain disorders (such as ALL or AML) will require immediate admission and chemotherapy, while others will be followed up with regular physical examination and blood tests.Often, surgery is attempted to remove a tumor entirely. This is only feasible when there is some degree of certainty that the tumor can in fact be removed. When it is certain that parts will remain, curative surgery is often impossible, e.g. when there are metastases elsewhere, or when the tumor has invaded a structure that cannot be operated upon without risking the patient's life. Occasionally surgery can improve survival even if not all tumour tissue has been removed; the procedure is referred to as "debulking" (i.e. reducing the overall amount of tumour tissue). Surgery is also used for the palliative treatment of some of cancers, e.g. to relieve biliary obstruction, or to relieve the problems associated with some cerebral tumors. The risks of surgery must be weighed against the benefits.
Chemotherapy and radiotherapy are used as a first-line radical therapy in a number of malignancies. They are also used for adjuvant therapy, i.e. when the macroscopic tumor has already been completely removed surgically but there is a reasonable statistical risk that it will recur. Chemotherapy and radiotherapy are commonly used for palliation, where disease is clearly incurable: in this situation the aim is to improve the quality of life and to prolong it.
Hormone manipulation is well established, particularly in the treatment of breast and prostate cancer.
There is currently a rapid expansion in the use of monoclonal antibody treatments, notably for lymphoma (Rituximab), and breast cancer (Trastuzumab).
Vaccine and other immunotherapies are the subject of intensive research.
Palliative care
Approximately 50% of all cancer cases in the Western world can be treated to remission with radical treatment. For pediatric patients, that number is much higher. A large number of cancer patients will die from the disease, and a significant proportion of patients with incurable cancer will die of other causes. There may be ongoing issues with symptom control associated with progressive cancer, and also with the treatment of the disease. These problems may include pain, nausea, anorexia, fatigue, immobility, and depression. Not all issues are strictly physical: personal dignity may be affected. Moral and spiritual issues are also important.While many of these problems fall within the remit of the oncologist, palliative care has matured into a separate, closely allied speciality to address the problems associated with advanced disease. Palliative care is an essential part of the multidisciplinary cancer care team. Palliative care services may be less hospital-based than oncology, with nurses and doctors who are able to visit the patient at home.
Ethical issues
There are a number of recurring ethical questions and dilemmas in oncological practice. These include:- What information to give the patient regarding disease extent/progression/prognosis.
- Entry into clinical trials, especially in the face of terminal illness.
- Withdrawal of active treatment.
- "Do Not Resuscitate" orders and other end of life issues.
Progress and research
There is a tremendous amount of research being conducted on all frontiers of oncology, ranging from cancer cell biology, radiation therapy to chemotherapy treatment regimens and optimal palliative care and pain relief. In the past decade, the advent of next-generation sequencing and whole-genome sequencing has completely changed our understanding of cancers. Identification of novel genetic/molecular markers will dramatically change how we diagnose and treat cancer, which will pave the way for personalized medicine.Therapeutic trials often involve patients from many different hospitals in a particular region. In the UK, patients are often enrolled in large studies coordinated by Cancer Research UK (CRUK),[3] Medical Research Council (MRC),[4] the European Organisation for Research and Treatment of Cancer (EORTC)[5] or the National Cancer Research Network (NCRN).[6]
Specialties
There are several sub-specialties within oncology. Moreover, oncologists often develop an interest and expertise in the management of particular types of cancer.Oncologists may be divided on the basis of the type of treatment provided or whether their role is primarily diagnostic.
- Radiology: localize, stage and often perform image-guided biopsy in order to obtain the tissue for preliminary diagnosis.
- Anatomical pathology: render the final diagnosis and prognosis of cancer, in order to guide treatment by oncologists.
- Radiation oncology: treatment primarily with radiation, a process called radiotherapy.[7]
- Surgical oncology: surgeons who specialize in tumor removal.[7]
- Medical oncology: treatment primarily with drugs, that is, pharmacotherapy, which includes chemotherapy, hormonal therapy, and targeted therapy.[7][8]
- Gynecologic oncology: focuses on cancers of the female reproductive system.
- Pediatric oncology: concerned with the treatment of cancer in children
In most countries it is now common that patients are treated by a multidisciplinary team. These teams meet on a regular basis and discuss the patients under their care. These teams consist of the medical oncologist, a clinical oncologist or radiotherapist, a surgeon (sometimes there is a second reconstructive surgeon), a radiologist, a pathologist, an organ specific specialist such as a gynecologist or dermatologist, and sometimes the general practitioner is also involved. These disease oriented teams are sometimes in conflict with the general organisation and operation in hospitals. Historically hospitals are organised in an organ or technique specific manner.
Multidisciplinary teams operate over these borders and it is sometimes difficult to define who is in charge.
In veterinary medicine, veterinary oncology is the sub-specialty that deals with cancer diagnosis and treatment in animals.
Subscribe to:
Posts (Atom)
Effects of climate change on the water cycle
From Wikipedia, the free encyclopedia https://en.wikipedia.org/wiki/Effects_of_climate_change_on_the_water_cycle ...

-
From Wikipedia, the free encyclopedia Ward Cunningham , inventor of the wiki A wiki is a website on whi...
-
From Wikipedia, the free encyclopedia Islamic State of Iraq and the Levant الدولة الإسلامية في العراق والشام ( ...
-
From Wikipedia, the free encyclopedia A reproduction of the palm -leaf manuscript in Siddham script ...