From Wikipedia, the free encyclopedia
Neural coding is a
neuroscience field concerned with characterising the hypothetical relationship between the
stimulus and the individual or ensemble neuronal responses and the relationship among the
electrical activity of the neurons in the ensemble. Based on the theory that
sensory and other information is represented in the
brain by
networks of neurons, it is thought that
neurons can encode both
digital and
analog information.
Neurons are remarkable among the
cells
of the body in their ability to propagate signals rapidly over large
distances. They do this by generating characteristic electrical pulses
called
action potentials:
voltage spikes that can travel down nerve fibers. Sensory neurons
change their activities by firing sequences of action potentials in
various temporal patterns, with the presence of external sensory
stimuli, such as
light,
sound,
taste,
smell and
touch.
It is known that information about the stimulus is encoded in this
pattern of action potentials and transmitted into and around the brain.
Although action potentials can vary somewhat in duration,
amplitude
and shape, they are typically treated as identical stereotyped events
in neural coding studies. If the brief duration of an action potential
(about 1ms) is ignored, an action potential sequence, or spike train,
can be characterized simply by a series of
all-or-none point events in time. The lengths of interspike intervals (
ISIs) between two successive spikes in a spike train often vary, apparently randomly.
The study of neural coding involves measuring and characterizing how
stimulus attributes, such as light or sound intensity, or motor actions,
such as the direction of an arm movement, are represented by neuron
action potentials or spikes. In order to describe and analyze neuronal
firing,
statistical methods and methods of
probability theory and stochastic
point processes have been widely applied.
With the development of large-scale neural recording and decoding
technologies, researchers have begun to crack the neural code and have
already provided the first glimpse into the real-time neural code as
memory is formed and recalled in the hippocampus, a brain region known
to be central for memory formation. Neuroscientists have initiated several large-scale brain decoding projects.
Encoding and decoding
The
link between stimulus and response can be studied from two opposite
points of view. Neural encoding refers to the map from stimulus to
response. The main focus is to understand how neurons respond to a wide
variety of stimuli, and to construct models that attempt to predict
responses to other stimuli.
Neural decoding
refers to the reverse map, from response to stimulus, and the challenge
is to reconstruct a stimulus, or certain aspects of that stimulus, from
the spike sequences it evokes.
Hypothesized coding schemes
A
sequence, or 'train', of spikes may contain information based on
different coding schemes. In motor neurons, for example, the strength at
which an innervated muscle is contracted depends solely on the 'firing
rate', the average number of spikes per unit time (a 'rate code'). At
the other end, a complex '
temporal code' is based on the precise timing of single spikes. They may be locked to an external stimulus such as in the visual and
auditory system or be generated intrinsically by the neural circuitry.
Whether neurons use rate coding or temporal coding is a topic of
intense debate within the neuroscience community, even though there is
no clear definition of what these terms mean. In one theory, termed
"neuroelectrodynamics", the following coding schemes are all considered
to be epiphenomena, replaced instead by molecular changes reflecting the
spatial distribution of
electric fields within neurons as a result of the broad
electromagnetic spectrum of action potentials, and manifested in information as
spike directivity.
Rate coding
The rate coding model of
neuronal firing communication states that as the intensity of a stimulus increases, the
frequency or rate of
action potentials, or "spike firing", increases. Rate coding is sometimes called frequency coding.
Rate coding is a traditional coding scheme, assuming that most,
if not all, information about the stimulus is contained in the firing
rate of the neuron. Because the sequence of action potentials generated
by a given stimulus varies from trial to trial, neuronal responses are
typically treated statistically or probabilistically. They may be
characterized by firing rates, rather than as specific spike sequences.
In most sensory systems, the firing rate increases, generally
non-linearly, with increasing stimulus intensity.
Any information possibly encoded in the temporal structure of the spike
train is ignored. Consequently, rate coding is inefficient but highly
robust with respect to the ISI '
noise'.
During rate coding, precisely calculating firing rate is very
important. In fact, the term "firing rate" has a few different
definitions, which refer to different averaging procedures, such as an
average over time or an average over several repetitions of experiment.
In rate coding, learning is based on activity-dependent synaptic weight modifications.
Rate coding was originally shown by
ED Adrian and
Y Zotterman in 1926. In this simple experiment different weights were hung from a
muscle.
As the weight of the stimulus increased, the number of spikes recorded
from sensory nerves innervating the muscle also increased. From these
original experiments, Adrian and Zotterman concluded that action
potentials were unitary events, and that the frequency of events, and
not individual event magnitude, was the basis for most inter-neuronal
communication.
In the following decades, measurement of firing rates became a
standard tool for describing the properties of all types of sensory or
cortical
neurons, partly due to the relative ease of measuring rates
experimentally. However, this approach neglects all the information
possibly contained in the exact timing of the spikes. During recent
years, more and more experimental evidence has suggested that a
straightforward firing rate concept based on temporal averaging may be
too simplistic to describe brain activity.
Spike-count rate
The
spike-count rate, also referred to as temporal average, is obtained by
counting the number of spikes that appear during a trial and dividing by
the duration of trial. The length T of the time window is set by the
experimenter and depends on the type of neuron recorded from and to the
stimulus. In practice, to get sensible averages, several spikes should
occur within the time window. Typical values are T = 100 ms or T = 500
ms, but the duration may also be longer or shorter.
The spike-count rate can be determined from a single trial, but
at the expense of losing all temporal resolution about variations in
neural response during the course of the trial. Temporal averaging can
work well in cases where the stimulus is constant or slowly varying and
does not require a fast reaction of the
organism
— and this is the situation usually encountered in experimental
protocols. Real-world input, however, is hardly stationary, but often
changing on a fast time scale. For example, even when viewing a static
image, humans perform
saccades, rapid changes of the direction of gaze. The image projected onto the retinal
photoreceptors changes therefore every few hundred milliseconds.
Despite its shortcomings, the concept of a spike-count rate code is widely used not only in experiments, but also in models of
neural networks.
It has led to the idea that a neuron transforms information about a
single input variable (the stimulus strength) into a single continuous
output variable (the firing rate).
There is a growing body of evidence that in
Purkinje neurons, at least, information is not simply encoded in firing but also in the timing and duration of non-firing, quiescent periods.
Time-dependent firing rate
The time-dependent firing rate is defined as the average number of
spikes (averaged over trials) appearing during a short interval between
times t and t+Δt, divided by the duration of the interval. It works for
stationary as well as for time-dependent stimuli. To experimentally
measure the time-dependent firing rate, the experimenter records from a
neuron while stimulating with some input sequence. The same stimulation
sequence is repeated several times and the neuronal response is reported
in a
Peri-Stimulus-Time Histogram
(PSTH). The time t is measured with respect to the start of the
stimulation sequence. The Δt must be large enough (typically in the
range of one or a few milliseconds) so there are sufficient number of
spikes within the interval to obtain a reliable estimate of the average.
The number of occurrences of spikes n
K(t;t+Δt) summed over
all repetitions of the experiment divided by the number K of repetitions
is a measure of the typical activity of the neuron between time t and
t+Δt. A further division by the interval length Δt yields time-dependent
firing rate r(t) of the neuron, which is equivalent to the spike
density of PSTH.
For sufficiently small Δt, r(t)Δt is the average number of spikes
occurring between times t and t+Δt over multiple trials. If Δt is
small, there will never be more than one spike within the interval
between t and t+Δt on any given trial. This means that r(t)Δt is also
the
fraction of trials on which a spike occurred between those times. Equivalently, r(t)Δt is the
probability that a spike occurs during this time interval.
As an experimental procedure, the time-dependent firing rate
measure is a useful method to evaluate neuronal activity, in particular
in the case of time-dependent stimuli. The obvious problem with this
approach is that it can not be the coding scheme used by neurons in the
brain. Neurons can not wait for the stimuli to repeatedly present in an
exactly same manner before generating response.
Nevertheless, the experimental time-dependent firing rate measure
can make sense, if there are large populations of independent neurons
that receive the same stimulus. Instead of recording from a population
of N neurons in a single run, it is experimentally easier to record from
a single neuron and average over N repeated runs. Thus, the
time-dependent firing rate coding relies on the implicit assumption that
there are always populations of neurons.
Temporal coding
When precise spike timing or high-frequency firing-rate
fluctuations are found to carry information, the neural code is often identified as a temporal code.
A number of studies have found that the temporal resolution of the
neural code is on a millisecond time scale, indicating that precise
spike timing is a significant element in neural coding.
Such codes, that communicate via the time between spikes are referred
to as interpulse interval codes, and have been supported by recent
studies.
Neurons exhibit high-frequency fluctuations of firing-rates which
could be noise or could carry information. Rate coding models suggest
that these irregularities are noise, while temporal coding models
suggest that they encode information. If the nervous system only used
rate codes to convey information, a more consistent, regular firing rate
would have been evolutionarily advantageous, and neurons would have
utilized this code over other less robust options.
Temporal coding supplies an alternate explanation for the “noise,"
suggesting that it actually encodes information and affects neural
processing. To model this idea, binary symbols can be used to mark the
spikes: 1 for a spike, 0 for no spike. Temporal coding allows the
sequence 000111000111 to mean something different from 001100110011,
even though the mean firing rate is the same for both sequences, at 6
spikes/10 ms. Until recently, scientists had put the most emphasis on rate encoding as an explanation for
post-synaptic potential patterns. However, functions of the brain are more temporally precise than the use of only rate encoding seems to allow
[citation needed].
In other words, essential information could be lost due to the
inability of the rate code to capture all the available information of
the spike train. In addition, responses are different enough between
similar (but not identical) stimuli to suggest that the distinct
patterns of spikes contain a higher volume of information than is
possible to include in a rate code.
Temporal codes employ those features of the spiking activity that
cannot be described by the firing rate. For example, time to first
spike after the stimulus onset, characteristics based on the second and
higher statistical
moments of the ISI
probability distribution, spike randomness, or precisely timed groups of spikes (temporal patterns) are candidates for temporal codes.
As there is no absolute time reference in the nervous system, the
information is carried either in terms of the relative timing of spikes
in a population of neurons or with respect to an
ongoing brain oscillation. One way in which temporal codes are decoded, in presence of
neural oscillations, is that spikes occurring at specific phases of an oscillatory cycle are more effective in depolarizing the
post-synaptic neuron.
The temporal structure of a spike train or firing rate evoked by a
stimulus is determined both by the dynamics of the stimulus and by the
nature of the neural encoding process. Stimuli that change rapidly tend
to generate precisely timed spikes and rapidly changing firing rates no
matter what neural coding strategy is being used. Temporal coding refers
to temporal precision in the response that does not arise solely from
the dynamics of the stimulus, but that nevertheless relates to
properties of the stimulus. The interplay between stimulus and encoding
dynamics makes the identification of a temporal code difficult.
In temporal coding, learning can be explained by activity-dependent synaptic delay modifications. The modifications can themselves depend not only on spike rates (rate
coding) but also on spike timing patterns (temporal coding), i.e., can
be a special case of
spike-timing-dependent plasticity.
The issue of temporal coding is distinct and independent from the
issue of independent-spike coding. If each spike is independent of all
the other spikes in the train, the temporal character of the neural code
is determined by the behavior of time-dependent firing rate r(t). If
r(t) varies slowly with time, the code is typically called a rate code,
and if it varies rapidly, the code is called temporal.
Temporal coding in sensory systems
For
very brief stimuli, a neuron's maximum firing rate may not be fast
enough to produce more than a single spike. Due to the density of
information about the abbreviated stimulus contained in this single
spike, it would seem that the timing of the spike itself would have to
convey more information than simply the average frequency of action
potentials over a given period of time. This model is especially
important for
sound localization,
which occurs within the brain on the order of milliseconds. The brain
must obtain a large quantity of information based on a relatively short
neural response. Additionally, if low firing rates on the order of ten
spikes per second must be distinguished from arbitrarily close rate
coding for different stimuli, then a neuron trying to discriminate these
two stimuli may need to wait for a second or more to accumulate enough
information. This is not consistent with numerous organisms which are
able to discriminate between stimuli in the time frame of milliseconds,
suggesting that a rate code is not the only model at work.
To account for the fast encoding of visual stimuli, it has been
suggested that neurons of the retina encode visual information in the
latency time between stimulus onset and first action potential, also
called latency to first spike.
This type of temporal coding has been shown also in the auditory and
somato-sensory system. The main drawback of such a coding scheme is its
sensitivity to intrinsic neuronal fluctuations. In the
primary visual cortex
of macaques, the timing of the first spike relative to the start of the
stimulus was found to provide more information than the interval
between spikes. However, the interspike interval could be used to encode
additional information, which is especially important when the spike
rate reaches its limit, as in high-contrast situations. For this reason,
temporal coding may play a part in coding defined edges rather than
gradual transitions.
The mammalian
gustatory system
is useful for studying temporal coding because of its fairly distinct
stimuli and the easily discernible responses of the organism.
Temporally encoded information may help an organism discriminate
between different tastants of the same category (sweet, bitter, sour,
salty, umami) that elicit very similar responses in terms of spike
count. The temporal component of the pattern elicited by each tastant
may be used to determine its identity (e.g., the difference between two
bitter tastants, such as quinine and denatonium). In this way, both rate
coding and temporal coding may be used in the gustatory system – rate
for basic tastant type, temporal for more specific differentiation.
Research on mammalian gustatory system has shown that there is an
abundance of information present in temporal patterns across populations
of neurons, and this information is different from that which is
determined by rate coding schemes. Groups of neurons may synchronize in
response to a stimulus. In studies dealing with the front cortical
portion of the brain in primates, precise patterns with short time
scales only a few milliseconds in length were found across small
populations of neurons which correlated with certain information
processing behaviors. However, little information could be determined
from the patterns; one possible theory is they represented the
higher-order processing taking place in the brain.
As with the visual system, in
mitral/tufted cells in the
olfactory bulb
of mice, first-spike latency relative to the start of a sniffing action
seemed to encode much of the information about an odor. This strategy
of using spike latency allows for rapid identification of and reaction
to an odorant. In addition, some mitral/tufted cells have specific
firing patterns for given odorants. This type of extra information could
help in recognizing a certain odor, but is not completely necessary, as
average spike count over the course of the animal's sniffing was also a
good identifier.
Along the same lines, experiments done with the olfactory system of
rabbits showed distinct patterns which correlated with different subsets
of odorants, and a similar result was obtained in experiments with the
locust olfactory system.
Temporal coding applications
The
specificity of temporal coding requires highly refined technology to
measure informative, reliable, experimental data. Advances made in
optogenetics
allow neurologists to control spikes in individual neurons, offering
electrical and spatial single-cell resolution. For example, blue light
causes the light-gated ion channel
channelrhodopsin
to open, depolarizing the cell and producing a spike. When blue light
is not sensed by the cell, the channel closes, and the neuron ceases to
spike. The pattern of the spikes matches the pattern of the blue light
stimuli. By inserting channelrhodopsin gene sequences into mouse DNA,
researchers can control spikes and therefore certain behaviors of the
mouse (e.g., making the mouse turn left).
Researchers, through optogenetics, have the tools to effect different
temporal codes in a neuron while maintaining the same mean firing rate,
and thereby can test whether or not temporal coding occurs in specific
neural circuits.
Optogenetic technology also has the potential to enable the
correction of spike abnormalities at the root of several neurological
and psychological disorders.
If neurons do encode information in individual spike timing patterns,
key signals could be missed by attempting to crack the code while
looking only at mean firing rates.
Understanding any temporally encoded aspects of the neural code and
replicating these sequences in neurons could allow for greater control
and treatment of neurological disorders such as
depression,
schizophrenia, and
Parkinson's disease.
Regulation of spike intervals in single cells more precisely controls
brain activity than the addition of pharmacological agents
intravenously.
Phase-of-firing code
Phase-of-firing code is a neural coding scheme that combines the
spike count code with a time reference based on
oscillations.
This type of code takes into account a time label for each spike
according to a time reference based on phase of local ongoing
oscillations at low or high frequencies.
It has been shown that neurons in some cortical sensory areas
encode rich naturalistic stimuli in terms of their spike times relative
to the phase of ongoing network oscillatory fluctuations, rather than
only in terms of their spike count. The
local field potential
signals reflect population (network) oscillations. The phase-of-firing
code is often categorized as a temporal code although the time label
used for spikes (i.e. the network oscillation phase) is a low-resolution
(coarse-grained) reference for time. As a result, often only four
discrete values for the phase are enough to represent all the
information content in this kind of code with respect to the phase of
oscillations in low frequencies. Phase-of-firing code is loosely based
on the
phase precession phenomena observed in place cells of the
hippocampus.
Another feature of this code is that neurons adhere to a preferred
order of spiking between a group of sensory neurons, resulting in firing
sequence.
Phase code has been shown in visual cortex to involve also high-frequency oscillations.
Within a cycle of gamma oscillation, each neuron has its own preferred
relative firing time. As a result, an entire population of neurons
generates a firing sequence that has a duration of up to about 15 ms.
Population coding
Population
coding is a method to represent stimuli by using the joint activities
of a number of neurons. In population coding, each neuron has a
distribution of responses over some set of inputs, and the responses of
many neurons may be combined to determine some value about the inputs.
From the theoretical point of view, population coding is one of a
few mathematically well-formulated problems in neuroscience. It grasps
the essential features of neural coding and yet is simple enough for
theoretic analysis.
Experimental studies have revealed that this coding paradigm is widely
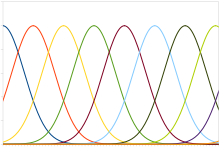
used in the sensor and motor areas of the brain. For example, in the
visual area
medial temporal (MT), neurons are tuned to the moving direction. In response to an object moving in a particular direction, many neurons in MT fire with a noise-corrupted and
bell-shaped
activity pattern across the population. The moving direction of the
object is retrieved from the population activity, to be immune from the
fluctuation existing in a single neuron’s signal.
In one classic example in the primary motor cortex, Apostolos
Georgopoulos and colleagues trained monkeys to move a joystick towards a
lit target.
They found that a single neuron would fire for multiple target
directions. However it would fire fastest for one direction and more
slowly depending on how close the target was to the neuron's 'preferred'
direction.
Kenneth Johnson originally derived that if each neuron represents
movement in its preferred direction, and the vector sum of all neurons
is calculated (each neuron has a firing rate and a preferred direction),
the sum points in the direction of motion. In this manner, the
population of neurons codes the signal for the motion. This particular
population code is referred to as
population vector
coding. This particular study divided the field of motor physiologists
between Evarts' "upper motor neuron" group, which followed the
hypothesis that motor cortex neurons contributed to control of single
muscles, and the Georgopoulos group studying the representation of
movement directions in cortex.
The Johns Hopkins University Neural Encoding laboratory led by
Murray Sachs and Eric Young developed place-time population codes,
termed the Averaged-Localized-Synchronized-Response (ALSR) code
for neural representation of auditory acoustic stimuli. This exploits
both the place or tuning within the auditory nerve, as well as the
phase-locking within each nerve fiber Auditory nerve.
The first ALSR representation was for steady-state vowels;
ALSR representations of pitch and formant frequencies in complex, non-steady state stimuli
were demonstrated for voiced-pitch and formant representations in consonant-vowel syllables.
The advantage of such representations is that global features such as
pitch or formant transition profiles can be represented as global
features across the entire nerve simultaneously via both
rate and place coding.
Population coding has a number of other advantages as well, including reduction of uncertainty due to neuronal
variability
and the ability to represent a number of different stimulus attributes
simultaneously. Population coding is also much faster than rate coding
and can reflect changes in the stimulus conditions nearly
instantaneously.
Individual neurons in such a population typically have different but
overlapping selectivities, so that many neurons, but not necessarily
all, respond to a given stimulus.
Typically an encoding function has a peak value such that
activity of the neuron is greatest if the perceptual value is close to
the peak value, and becomes reduced accordingly for values less close to
the peak value.
It follows that the actual perceived value can be reconstructed
from the overall pattern of activity in the set of neurons. The
Johnson/Georgopoulos vector coding is an example of simple averaging. A
more sophisticated mathematical technique for performing such a
reconstruction is the method of
maximum likelihood based on a multivariate distribution of the neuronal responses. These models can assume independence, second order correlations, or even more detailed dependencies such as higher order
maximum entropy models or
copulas.
Correlation coding
The correlation coding model of
neuronal firing claims that correlations between
action potentials,
or "spikes", within a spike train may carry additional information
above and beyond the simple timing of the spikes. Early work suggested
that correlation between spike trains can only reduce, and never
increase, the total
mutual information present in the two spike trains about a stimulus feature. However, this was later demonstrated to be incorrect. Correlation
structure can increase information content if noise and signal
correlations are of opposite sign.
Correlations can also carry information not present in the average
firing rate of two pairs of neurons. A good example of this exists in
the pentobarbital-anesthetized marmoset auditory cortex, in which a pure
tone causes an increase in the number of correlated spikes, but not an
increase in the mean firing rate, of pairs of neurons.
Independent-spike coding
The independent-spike coding model of
neuronal firing claims that each individual
action potential, or "spike", is independent of each other spike within the
spike train.
Position coding
Plot of typical position coding
A typical population code involves neurons with a Gaussian tuning
curve whose means vary linearly with the stimulus intensity, meaning
that the neuron responds most strongly (in terms of spikes per second)
to a stimulus near the mean. The actual intensity could be recovered as
the stimulus level corresponding to the mean of the neuron with the
greatest response. However, the noise inherent in neural responses
means that a maximum likelihood estimation function is more accurate.
Neural responses are noisy and unreliable.
This type of code is used to encode continuous variables such as
joint position, eye position, color, or sound frequency. Any individual
neuron is too noisy to faithfully encode the variable using rate coding,
but an entire population ensures greater fidelity and precision. For a
population of unimodal tuning curves, i.e. with a single peak, the
precision typically scales linearly with the number of neurons. Hence,
for half the precision, half as many neurons are required. In contrast,
when the tuning curves have multiple peaks, as in
grid cells
that represent space, the precision of the population can scale
exponentially with the number of neurons. This greatly reduces the
number of neurons required for the same precision.
Sparse coding
The
sparse code is when each item is encoded by the strong activation of a
relatively small set of neurons. For each item to be encoded, this is a
different subset of all available neurons. In contrast to sensor-sparse
coding, sensor-dense coding implies that all information from possible
sensor locations is known.
As a consequence, sparseness may be focused on temporal
sparseness ("a relatively small number of time periods are active") or
on the sparseness in an activated population of neurons. In this latter
case, this may be defined in one time period as the number of activated
neurons relative to the total number of neurons in the population. This
seems to be a hallmark of neural computations since compared to
traditional computers, information is massively distributed across
neurons. A major result in neural coding from Olshausen and Field is that
sparse coding of natural images produces
wavelet-like
oriented filters that resemble the receptive fields of simple cells in
the visual cortex. The capacity of sparse codes may be increased by
simultaneous use of temporal coding, as found in the locust olfactory
system.
Given a potentially large set of input patterns, sparse coding algorithms (e.g.
Sparse Autoencoder)
attempt to automatically find a small number of representative patterns
which, when combined in the right proportions, reproduce the original
input patterns. The sparse coding for the input then consists of those
representative patterns. For example, the very large set of English
sentences can be encoded by a small number of symbols (i.e. letters,
numbers, punctuation, and spaces) combined in a particular order for a
particular sentence, and so a sparse coding for English would be those
symbols.
Linear generative model
Most models of sparse coding are based on the linear generative model. In this model, the symbols are combined in a
linear fashion to approximate the input.
More formally, given a k-dimensional set of real-numbered input vectors

, the goal of sparse coding is to determine n k-dimensional
basis vectors 
along with a
sparse n-dimensional vector of weights or coefficients

for each input vector, so that a linear combination of the basis
vectors with proportions given by the coefficients results in a close
approximation to the input vector:

.
The codings generated by algorithms implementing a linear generative model can be classified into codings with
soft sparseness and those with
hard sparseness. These refer to the distribution of basis vector coefficients for typical inputs. A coding with soft sparseness has a smooth
Gaussian-like
distribution, but peakier than Gaussian, with many zero values, some
small absolute values, fewer larger absolute values, and very few very
large absolute values. Thus, many of the basis vectors are active. Hard
sparseness, on the other hand, indicates that there are many zero
values,
no or
hardly any small absolute values, fewer
larger absolute values, and very few very large absolute values, and
thus few of the basis vectors are active. This is appealing from a
metabolic perspective: less energy is used when fewer neurons are
firing.
Another measure of coding is whether it is
critically complete or
overcomplete.
If the number of basis vectors n is equal to the dimensionality k of
the input set, the coding is said to be critically complete. In this
case, smooth changes in the input vector result in abrupt changes in the
coefficients, and the coding is not able to gracefully handle small
scalings, small translations, or noise in the inputs. If, however, the
number of basis vectors is larger than the dimensionality of the input
set, the coding is
overcomplete. Overcomplete codings smoothly interpolate between input vectors and are robust under input noise. The human primary
visual cortex
is estimated to be overcomplete by a factor of 500, so that, for
example, a 14 x 14 patch of input (a 196-dimensional space) is coded by
roughly 100,000 neurons.
Biological evidence
Sparse coding
may be a general strategy of neural systems to augment memory capacity.
To adapt to their environments, animals must learn which stimuli are
associated with rewards or punishments and distinguish these reinforced
stimuli from similar but irrelevant ones. Such task requires
implementing stimulus-specific
associative memories in which only a few neurons out of a
population respond to any given stimulus and each neuron responds to only a few stimuli out of all possible stimuli.
Theoretical work on
Sparse distributed memory
has suggested that sparse coding increases the capacity of associative
memory by reducing overlap between representations. Experimentally,
sparse representations of sensory information have been observed in many
systems, including vision, audition, touch, and olfaction.
However, despite the accumulating evidence for widespread sparse coding
and theoretical arguments for its importance, a demonstration that
sparse coding improves the stimulus-specificity of associative memory
has been lacking until recently.
Some progress has been made in 2014 by
Gero Miesenböck's lab at the
University of Oxford analyzing
Drosophila Olfactory system.
In Drosophila, sparse odor coding by the
Kenyon cells of the
mushroom body is thought to generate a large number of precisely addressable locations for the storage of odor-specific memories. Lin et al. demonstrated that sparseness is controlled by a negative feedback circuit between Kenyon cells and the
GABAergic
anterior paired lateral (APL) neuron. Systematic activation and
blockade of each leg of this feedback circuit show that Kenyon cells
activate APL and APL inhibits Kenyon cells. Disrupting the Kenyon
cell-APL feedback loop decreases the sparseness of Kenyon cell odor
responses, increases inter-odor correlations, and prevents flies from
learning to discriminate similar, but not dissimilar, odors. These
results suggest that feedback inhibition suppresses Kenyon cell activity
to maintain sparse, decorrelated odor coding and thus the
odor-specificity of memories.






![r_{{p}}=\left|\left[e_{{p}}-\sum _{{j=1,j\neq p}}^{{n}}k_{{pj}}\left|r_{{j}}-r_{{pj}}^{{o}}\right|\right]\right|](https://wikimedia.org/api/rest_v1/media/math/render/svg/0d90ffed5e4a6921f3feeb1ff1ff58ca526526b5)