| Vitamin E | |
|---|---|
| Drug class | |

The RRR alpha-tocopherol form of vitamin E
| |
| Class identifiers | |
| Use | Vitamin E deficiency, antioxidant |
| ATC code | A11HA03 |
| Biological target | Reactive oxygen species |
| Clinical data | |
| Drugs.com | MedFacts Natural Products |
| External links | |
| MeSH | D014810 |
Vitamin E is a group of eight fat soluble compounds that include four tocopherols and four tocotrienols. Vitamin E deficiency, which is rare and usually due to an underlying problem with digesting dietary fat rather than from a diet low in vitamin E, can cause nerve problems. The crucial function played by Vitamin E that makes it a vitamin is poorly understood, but may involve antioxidant functions in cell membranes. Other theories hold that vitamin E – specifically the RRR stereoisomer of alpha-tocopherol – act by controlling gene expression and cell signal transduction.
Worldwide, government organizations recommend adults consume in the range of 7 to 15 mg per day. As of 2016, consumption was below recommendations according to a worldwide summary of more than one hundred studies that reported a median dietary intake of 6.2 mg per day for alpha-tocopherol. Research with alpha-tocopherol as a dietary supplement, with daily amounts as high as 2000 mg per day, has had mixed results. Population studies suggested that people who consumed foods with more vitamin E, or who chose on their own to consume a vitamin E dietary supplement, had lower incidence of cardiovascular diseases, cancer, dementia, and other diseases, but placebo-controlled clinical trials could not always replicate these findings, and there were some indications that vitamin E supplementation actually was associated with a modest increase in all-cause mortality. As of 2017, vitamin E continues to be a topic of active clinical research. Although people commonly apply Vitamin E oil to their skin to try to improve wound healing and reduce scar tissue, reviews have repeatedly concluded that there is no good evidence that this is helpful.
Both the tocopherols and tocotrienols occur in α (alpha), β (beta), γ (gamma) and δ (delta) forms, as determined by the number and position of methyl groups on the chromanol ring. All eight of these vitamers feature a chromane double ring, with a hydroxyl group that can donate a hydrogen atom to reduce free radicals, and a hydrophobic side chain which allows for penetration into biological membranes. Of the many different forms of vitamin E, gamma-tocopherol (γ-tocopherol) is the most common form found in the North American diet, but alpha-tocopherol (α-tocopherol) is the most biologically active. Palm oil is a source of tocotrienols.
Vitamin E was discovered in 1922, isolated in 1935 and first synthesized in 1938. Because the vitamin activity was first identified as essential for fertilized eggs to result in live births (in rats), it was given the name "tocopherol" from Greek words meaning birth and to bear or carry. Alpha-tocopherol, either naturally extracted from plant oils or synthetic, is sold as a popular dietary supplement, either by itself or incorporated into a multivitamin product, and in oils or lotions for use on skin.
Functions
Tocopherols function by donating H atoms to radicals (X).
Vitamin E may have various roles as a vitamin. Many biological functions have been postulated, including a role as a fat-soluble antioxidant. In this role, vitamin E acts as a radical scavenger, delivering a hydrogen (H) atom to free radicals. At 323 kJ/mol, the O-H bond in tocopherols is about 10% weaker than in most other phenols. This weak bond allows the vitamin to donate a hydrogen atom to the peroxyl radical and other free radicals, minimizing their damaging effect. The thus-generated tocopheryl radical is recycled to tocopherol by a redox reaction with a hydrogen donor, such as vitamin C. As it is fat-soluble, vitamin E is incorporated into cell membranes, which are therefore protected from oxidative damage.
Vitamin E affects gene expression and is an enzyme activity regulator, such as for protein kinase C (PKC) – which plays a role in smooth muscle growth – with vitamin E participating in deactivation of PKC to inhibit smooth muscle growth.
Deficiency
Vitamin E deficiency is rare in humans, occurring as a consequence of
abnormalities in dietary fat absorption or metabolism rather than from a
diet low in vitamin E. One example of a genetic abnormality in metabolism is mutations of genes coding for alpha-tocopherol transfer protein
(α-TTP). Humans with this genetic defect exhibit a progressive
neurodegenerative disorder known as ataxia with vitamin E deficiency
(AVED) despite consuming normal amounts of vitamin E. Large amounts of
alpha-tocopherol as a dietary supplement are needed to compensate for
the lack of α-TTP. Vitamin E deficiency due to either malabsorption or metabolic anomaly can cause nerve problems due to poor conduction of electrical impulses along nerves due to changes in nerve membrane structure and function. In addition to ataxia, vitamin E deficiency can cause peripheral neuropathy, myopathies, retinopathy and impairment of immune responses.
Frequency of dietary supplement use
In
the United States vitamin E supplement use by female health
professionals was 16.1% in 1986, 46.2% in 1998, 44.3% in 2002, but
decreased to 19.8% in 2006. Similarly, for male health professionals,
rates for same years were 18.9%, 52.0%, 49.4% and 24.5%. The authors
theorized that declining use in these populations may have due to
publications of studies that showed either no benefits or negative
consequences from vitamin E supplements.
Within the US military services, vitamin prescriptions written for
active, reserve and retired military, and their dependents, were tracked
over years 2007-2011. Vitamin E prescriptions decreased by 53% while
vitamin C remained constant and vitamin D increased by 454%. A report on vitamin E sales volume in the US documented a 50% decrease between 2000 and 2006, with a cause attributed to a meta-analysis that had concluded that high-dosage vitamin E increased all-cause mortality.
Side effects
The U.S. Food and Nutrition Board set a Tolerable upper intake level (UL) at 1,000 mg (1,500 IU) per day derived from animal models that demonstrated bleeding at high doses. The European Food Safety Authority reviewed the same safety question and set a UL at 300 mg/day.
A meta-analysis of long-term clinical trials reported a non-significant
2% increase in all-cause mortality when alpha-tocopherol was the only
supplement used. The same meta-analysis reported a statistically
significant 3% increase for results when alpha-tocopherol was used by
itself or in combination with other nutrients (vitamin A, vitamin C,
beta-carotene, selenium).
Another meta-analysis reported a non-significant 1% increase in
all-cause mortality when alpha-tocopherol was the only supplement.
Subset analysis reported no difference between natural (plant extracted)
or synthetic alpha-tocopherol, or whether the amount used was less than
or more than 400 IU/day.
There are reports of vitamin E-induced allergic contact dermatitis from
use of vitamin-E derivatives such as tocopheryl linoleate and
tocopherol acetate in skin care products. Incidence is low despite
widespread use.
Drug interactions
The
amounts of alpha-tocopherol, other tocopherols and tocotrienols that
are components of dietary vitamin E, when consumed from foods, do not
appear to cause any interactions with drugs. Consumption of
alpha-tocopherol as a dietary supplement in amounts in excess of
300 mg/day may lead to interactions with aspirin, warfarin, tamoxifen and cyclosporine A in ways that alter function. For aspirin and warfarin, high amounts of vitamin E may potentiate anti-blood clotting action.
One small trial demonstrated that vitamin E at 400 mg/day reduced blood
concentration of the anti-breast cancer drug tamoxifen. In multiple
clinical trials, vitamin E lowered blood concentration of the
immuno-suppressant drug, cyclosporine A.
The US National Institutes of Health, Office of Dietary Supplements,
raises a concern that co-administration of vitamin E could counter the
mechanisms of anti-cancer radiation therapy and some types of
chemotherapy, and so advises against its use in these patient
populations. The references it cited reported instances of reduced
treatment adverse effects, but also poorer cancer survival, raising the
possibility of tumor protection from the intended oxidative damage by
the treatments.
Diet
Recommendations
The U.S. Institute of Medicine (renamed National Academy of Medicine in 2015) updated Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for vitamin E in 2000.
The EAR for vitamin E for women and men ages 14 and up is 12 mg/day.
The RDA is 15 mg/day. RDAs are higher than EARs so as to identify
amounts that will cover people with higher than average requirements.
For infants up to 12 months the Adequate Intake (AI) is 4–5 mg/day. As
for safety, Tolerable upper intake levels
(ULs) are set for vitamins and minerals when evidence is sufficient.
Hemorrhagic effects in rats were selected as the critical endpoint to
calculate the UL via starting with the
lowest-observed-adverse-effect-level (LOAEL) and processing that through
an uncertainty factor calculation. The end result was a UL set at
1000 mg/day. Collectively the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes (DRIs).
The European Food Safety Authority
(EFSA) refers to the collective set of information as Dietary Reference
Values, with Population Reference Intake (PRI) instead of RDA, and
Average Requirement instead of EAR. AI and UL defined the same as in
United States. For women and men ages 10 and older the PRIs are set at
11 and 13 mg/day, respectively. PRI for pregnancy is 11 mg/day, for
lactation 11 mg/day. For children ages 1–9 years the PRIs increase with
age from 6 to 9 mg/day. These PRIs are lower than the U.S. RDAs.
The European Food Safety Authority reviewed the same safety question
and set a UL at 300 mg/day. The EU used an effect on blood clotting as a
critical effect, identified that no adverse effects were observed in a
human trial as 540 mg/day, used an uncertainty factor of 2 to get to a
suggest UL of 270 mg/day, then rounded up to 300 mg/day.
The Japan National Institute of Health and Nutrition set lower
AIs than the U.S. RDAs or EU PRIs, and intermediate ULs: 6.5 mg/day
(females) and 7.0 mg/day (males) for adult AIs, and 650–700 mg/day
(females) and 750–900 mg/day (males) for adult ULs, amount depending on
age. India recommends an intake of 8–10 mg/day and does not set a UL. The World Health Organization recommends that adults consume 10 mg/day.
Consumption is below government recommendations. A worldwide
summary of more than one hundred studies reported a median dietary
intake of 6.2 mg/d for alpha-tocopherol. Government survey results in the U.S. reported average consumption for adult females at 8.4 mg/d and adult males 10.4 mg/d. Both are both below the RDA of 15 mg/day.
Food labeling
For
U.S. food and dietary supplement labeling purposes the amount in a
serving is expressed as a percent of Daily Value (%DV). For vitamin E
labeling purposes 100% of the Daily Value was 30 IU, but as of May 27,
2016 it was revised to 15 mg to bring it into agreement with the RDA. A table of the old and new adult Daily Values is provided at Reference Daily Intake.
The original deadline to be in compliance was July 28, 2018, but on
September 29, 2017 the FDA released a proposed rule that extended the
deadline to January 1, 2020 for large companies and January 1, 2021 for
small companies.
European Union regulations require that labels declare energy, protein,
fat, saturated fat, carbohydrates, sugars, and salt. Voluntary
nutrients may be shown if present in significant amounts. Instead of
Daily Values, amounts are shown as percent of Reference Intakes (RIs).
For vitamin E, 100% RI was set at 12 mg in 2011.
Sources
The
U.S. Department of Agriculture (USDA), Agricultural Research Services,
maintains a food composition database. The last major revision was
Release 28, September 2015. In addition to the naturally occurring
sources shown in the table, certain ready-to-eat cereals, infant formulas, liquid nutrition products and other foods are fortified with alpha-tocopherol.
| Plant source | Amount (mg / 100g) |
|---|---|
| Wheat germ oil | 150 |
| Hazelnut oil | 47 |
| Canola/rapeseed oil | 44 |
| Sunflower oil | 41.1 |
| Safflower oil | 34.1 |
| Almond oil | 39.2 |
| Grapeseed oil | 28.8 |
| Sunflower seed kernels | 26.1 |
| Almonds | 25.6 |
| Almond butter | 24.2 |
| Wheat germ | 19 |
| Plant source | Amount (mg / 100g) |
|---|---|
| Canola oil | 17.5 |
| Palm oil | 15.9 |
| Peanut oil | 15.7 |
| Margarine, tub | 15.4 |
| Hazelnuts | 15.3 |
| Corn oil | 14.8 |
| Olive oil | 14.3 |
| Soybean oil | 12.1 |
| Pine nuts | 9.3 |
| Peanut butter | 9.0 |
| Peanuts | 8.3 |
| Plant source | Amount (mg / 100g) |
|---|---|
| Popcorn | 5.0 |
| Pistachio nuts | 2.8 |
| Mayonnaise | 3.3 |
| Avocados | 2.6 |
| Spinach, raw | 2.0 |
| Asparagus | 1.5 |
| Broccoli | 1.4 |
| Cashew nuts | 0.9 |
| Bread | 0.2-0.3 |
| Rice, brown | 0.2 |
| Potato, Pasta | <0 .1="" span=""> |
| Animal source | Amount (mg / 100g) |
|---|---|
| Fish | 1.0-2.8 |
| Oysters | 1.7 |
| Butter | 1.6 |
| Cheese | 0.6-0.7 |
| Eggs | 1.1 |
| Chicken | 0.3 |
| Beef | 0.1 |
| Pork | 0.1 |
| Milk, whole | 0.1 |
| Milk, skim | 0.01 |
Supplements
Softgel capsules used for large amounts of vitamin E
Vitamin E is fat soluble, so dietary supplement products are usually
in the form of the vitamin dissolved in vegetable oil in a softgel
capsule. For alpha-tocopherol, amounts range from 100 to 1000 IU per
serving. Smaller amounts are incorporated into multi-vitamin/mineral
tablets. Gamma-tocopherol and tocotrienol supplements are also available
from dietary supplement companies. The latter are extracts from palm or
annatto oils.
Fortification
The World Health Organization does not have any recommendations for food fortification with vitamin E. The Food Fortification Initiative does not list any countries that have mandatory or voluntary programs for vitamin E.
Infant formulas have alpha-tocopherol as an ingredient. In some
countries, certain brands of ready-to-eat cereals, liquid nutrition
products and other foods have alpha-tocopherol as an added ingredient.
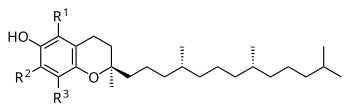
Chemistry
General chemical structure of tocopherols
RRR alpha-tocopherol; chiral points are where the three dashed lines connect to the side chain
The nutritional content of vitamin E is defined by equivalency to
100% RRR-configuration α-tocopherol activity. The molecules that
contribute α-tocopherol activity are four tocopherols and four
tocotrienols, within each group of four identified by the prefixes
alpha- (α-), beta- (β-), gamma- (γ-), and delta- (δ-). For
alpha(α)-tocopherol each of the three "R" sites has a methyl group (CH3)
attached. For beta(β)-tocopherol: R1 = methyl group, R2 = H, R3 =
methyl group. For gamma(γ)-tocopherol: R1 = H, R2 = methyl group, R3 =
methyl group. For delta(δ)-tocopherol: R1 = H, R2 = H, R3 = methyl
group. The same configurations exist for the tocotrienols, except that
the hydrophobic side chain has three carbon-carbon double bonds whereas
the tocopherols have a saturated side chain.
Stereoisomers
In
addition to distinguishing tocopherols and tocotrienols by position of
methyl groups, the tocopherols have a phytl tail with three chiral
points or centers that can have a right or left orientation. The
naturally occurring plant form of alpha-tocopherol is RRR-α-tocopherol,
also referred to as d-tocopherol, whereas the synthetic form
(all-racemic or all-rac vitamin E, also dl-tocopherol) is equal parts of eight stereoisomers
RRR, RRS, RSS, SSS, RSR, SRS, SRR and SSR with progressively decreasing
biological equivalency, so that 1.36 mg of dl-tocopherol is considered
equivalent to 1.0 mg of d-tocopherol, the natural form. Rephrased, the
synthetic has 73.5% of the potency of the natural.
| Form | Structure |
|---|---|
| alpha-Tocopherol | 
|
| beta-Tocopherol | 
|
| gamma-Tocopherol | |
| delta-Tocopherol |
Tocopherols
General chemical structure of tocotrienols.
alpha-Tocopherol is a lipid-soluble antioxidant functioning within the glutathione peroxidase pathway, and protecting cell membranes from oxidation by reacting with lipid radicals produced in the lipid peroxidation chain reaction. This removes the free radical intermediates and prevents the oxidation
reaction from continuing. The oxidized α-tocopheroxyl radicals produced
in this process may be recycled back to the active reduced form through
reduction by other antioxidants, such as ascorbate, retinol or ubiquinol. Other forms of vitamin E have their own unique properties; for example, γ-tocopherol is a nucleophile that can react with electrophilic mutagens.
Tocotrienols
The four tocotrienols
(alpha, beta, gamma, delta) are similar in structure to the four
tocopherols, with the main difference being that the former have
hydrophobic side chains with three carbon-carbon double bonds, whereas
the tocopherols have saturated side chains. For alpha(α)-tocotrienol each of the three "R" sites has a methyl group (CH3) attached. For beta(β)-tocotrienol: R1 = methyl group, R2 = H, R3 = methyl group. For gamma(γ)-tocotrienol: R1 = H, R2 = methyl group, R3 = methyl group. For delta(δ)-tocotrienol: R1 = H, R2 = H, R3 = methyl group. Palm oil is a good source of alpha and gamma tocotrienols.
Tocotrienols have only a single chiral center,
which exists at the 2' chromanol ring carbon, at the point where the
isoprenoid tail joins the ring. The other two corresponding centers in
the phytyl tail of the corresponding tocopherols do not exist as chiral
centers for tocotrienols due to unsaturation (C-C double bonds) at these
sites. Tocotrienols extracted from plants are always dextrorotatory stereoisomers, signified as d-tocotrienols. In theory, (levorotatory;
l-tocotrienol) forms of tocotrienols could exist as well, which would
have a 2S rather than 2R configuration at the molecules' single chiral
center, but unlike synthetic, dl-alpha-tocopherol, the marketed
tocotrienol dietary supplements are all d-tocotrienol extracts from palm or annatto oils. Preliminary clinical trials on dietary supplement tocotrienols indicate potential for anti-disease activity.
Metabolism
Tocotrienols
and tocopherols, the latter including the stereoisomers of synthetic
alpha-tocopherol, are absorbed from the intestinal lumen, incorporated
into chylomicrons, and secreted into the portal vein, leading to the liver. Absorption efficiency is estimated at 51% to 86%, and that applies to all of the vitamin E family–there is no discrimination among the vitamin E vitamers during absorption. Unabsorbed vitamin E is excreted via feces. Additionally, vitamin E is excreted by the liver via bile
into the intestinal lumin, where it will either be reabsorbed or
excreted via feces, and all of the vitamin E vitamers are metabolized
and then excreted via urine.
Upon reaching the liver, RRR-alpha-tocopherol is preferentially taken up by alpha-tocopherol transfer protein
(α-TTP). All other forms are degraded to
2'-carboxethyl-6-hydroxychromane (CEHC), a process that involves
truncating the phytic tail of the molecule, then either sulfated or
glycuronidated. This renders the molecules water-soluble and leads to
excretion via urine. Alpha-tocopherol is also degraded by the same
process, to 2,5,7,8-tetramethyl-2-(2 ′-carboxyethyl)-6-hydroxychromane
(α-CEHC), but more slowly because it is partially protected by α-TTP.
Large intakes of α-tocopherol result in increased urinary α-CEHC, so
this appears to be a means of disposing of excess vitamin E.
Alpha-tocopherol transfer protein is coded by the TTPA gene on
chromosome 8. The binding site for RRR-α-tocopherol is a hydrophobic
pocket with a lower affinity for beta-, gamma-, or delta-tocopherols, or
for the stereoisomers with an S configuration at the chiral 2 site.
Tocotrienols are also a poor fit because the double bonds in the phytic
tail create a rigid configuration that is a mismatch with the α-TTP
pocket.
A rare genetic defect of the TTPA gene results in people exhibiting a
progressive neurodegenerative disorder known as ataxia with vitamin E
deficiency (AVED) despite consuming normal amounts of vitamin E. Large
amounts of alpha-tocopherol as a dietary supplement are needed to
compensate for the lack of α-TTP
The role of α-TTP is to move α-tocopherol to the plasma membrane of
hepatocytes (liver cells), where in can be incorporated into newly
created very low density lipoprotein (VLDL) molecules. These convey
α-tocopherol to cells in the rest of the body. As an example of a result
of the preferential treatment, the US diet delivers approximately
70 mg/d of γ-tocopherol and plasma concentrations are on the order of
2–5 µmol/L; meanwhile. dietary α-tocopherol is about 7 mg/d but plasma
concentrations are in the range of 11–37 µmol/L.
Affinity of α-TTP for vitamin E vitamers
| Vitamin E compound | Affinity |
|---|---|
| RRR-αlpha-tocopherol | 100% |
| beta-tocopherol | 38% |
| gamma-tocopherol | 9% |
| delta-tocopherol | 2% |
| SSR-alpha-tocopherol | 11% |
| alpha-tocotrienol | 12% |
Testing for levels
A
worldwide summary of more than one hundred human studies reported a
median of 22.1 µmol/L for serum α-tocopherol, and defined α-tocopherol
deficiency as less than 12 µmol/L. It cited a recommendation that serum
α-tocopherol concentration be ≥30 µmol/L to optimize health benefits.
In contrast, the US Dietary Reference Intake text for vitamin E
concluded that a plasma concentration of 12 µmol/L was sufficient to
achieve normal ex vivo hydrogen peroxide-induced hemolysis. A 2014 review defined less than 9 µmol/L as deficient, 9-12 µmol/L as marginal and greater than 12 µmol/L as adequate.
Serum concentration increases with age. This is attributed to
fact that vitamin E circulates in blood incorporated into lipoproteins,
and serum lipoprotein concentrations increase with age. Infants and
young children have a higher risk of being below the deficiency
threshold.
Cystic fibrosis and other fat malabsorption conditions can result in
low serum vitamin E. Dietary supplements will raise serum vitamin E.
Synthesis
Biosynthesis
Photosynthesizing plants, algae and cyanobacteria
synthesize tocochromanols, the chemical family of compounds made up of
four tocopherols and four tocotrienols; in a nutrition context this
family is referred to as Vitamin E. Biosynthesis starts with formation
of the closed-ring part of the molecule as homogentisic acid (HGA). The
side chain is attached (saturated for tocopherols, polyunsaturated for tocotrienols).
The pathway for both is the same, so that gamma- is created and from
that alpha-, or delta- is created and from that the beta- compounds. Biosynthesis takes place in the plastids.
As to why plants synthesize tocochromanols, the major reason
appears to be for antioxidant activity. Different parts of plants, and
different species, are dominated by different tocochromamols. The
predominant form in leaves, and hence leafy green vegetables is
α-tocopherol. Location is in chloroplast membranes, this in close proximity to the photosynthetic process.
The function is to protect against damage from the ultraviolet
radiation of sunlight. Under normal growing conditions the presence of
α-tocopherol does not appear to be essential, as there are other
photo-protective compounds, and plant mutations that have lost the
ability to synthesize α-tocopherol demonstrate normal growth. However,
under stressed growing conditions such as drought, elevated temperature
or salt-induced oxidative stress, the plants' physiological status is
superior if it has the normal synthesis capacity.
Seeds are lipid-rich, to provide energy for germination and early growth. Tocochromanols protect the seed lipids from oxidizing and becoming rancid. The presence of tocochromanols extends seed longevity, and promotes successful germination and seedling growth.
Gamma-tocopherol dominates in seeds of most plant species, but there
are exceptions. For canola, corn and soy bean oils, there is more
γ-tocopherol than α-tocopherol, but for safflower, sunflower and olive
oils the reverse is true. Of the commonly used food oils, palm oil is unique in that tocotrienol content is higher than tocopherol content.
Seed tocochromanols content is also dependent on environmental
stressors. In almonds, for example, drought or elevated temperature
increase α-tocopherol and γ-tocopherol content of the nuts. The same
article mentions that drought increases the tocopherol content of
olives, and heat likewise for soybeans.
Industrial synthesis
Naturally
sourced d-alpha-tocopherol can be extracted and purified from seed
oils, or gamma-tocopherol can be extracted, purified, and methylated to
create d-alpha-tocopherol. In contrast to alpha-tocopherol extracted
from plants, which is also called d-alpha-tocopherol, industrial
synthesis creates dl-alpha-tocopherol. "It is synthesized from a mixture
of toluene and 2,3,5-trimethyl-hydroquinone that reacts with isophytol
to all-rac-alpha-tocopherol, using iron in the presence of hydrogen
chloride gas as catalyst. The reaction mixture obtained is filtered and
extracted with aqueous caustic soda. Toluene is removed by evaporation
and the residue (all rac-alpha-tocopherol) is purified by vacuum
distillation." Specification for the ingredient is more than 97% pure.
This synthetic dl-alpha-tocopherol has approximately 50% of the potency
of d-alpha-tocopherol. Manufacturers of dietary supplements and
fortified foods for humans or domesticated animals convert the phenol
form of the vitamin to an ester using either acetic acid or succinic acid
because the esters are more chemically stable, providing for a longer
shelf-life. The ester forms are de-esterified in the gut and absorbed as
free alpha-tocopherol.
History
Vitamin E was discovered in 1922 by Herbert McLean Evans and Katharine Scott Bishop and first isolated in a pure form by Evans and Gladys Anderson Emerson in 1935 at the University of California, Berkeley.
Because the vitamin activity was first identified as a dietary
fertility factor (in rats) it was given the name "tocopherol" from the
Greek words "τόκος" [tókos, birth], and "φέρειν", [phérein, to bear or
carry] meaning in sum "to carry a pregnancy," with the ending "-ol"
signifying its status as a chemical alcohol. George M. Calhoun,
Professor of Greek at the University of California, was credited with
helping with the naming process. Erhard Fernholz elucidated its structure in 1938 and shortly afterwards the same year, Paul Karrer and his team first synthesized it.
Nearly 50 years after the discovery of vitamin E an editorial in
the Journal of the American Medical Association titled "Vitamin in
search of a disease" read in part "...research revealed many of the
vitamin's secrets, but no certain therapeutic use and no definite
deficiency disease in man." The animal discovery experiments had been a
requirement for successful pregnancy, but no benefits were observed for
women prone to miscarriage. Evidence for vascular health was
characterized as unconvincing. The editorial closed with mention of some
preliminary human evidence for protection against hemolytic anemia in
young children.
A role for vitamin E in coronary heart disease had first been proposed in 1946. More cardiovascular work from the same research group followed, including a proposal that megadoses of vitamin E could slow down and even reverse the development of atherosclerosis.
However, a 2004 meta-analysis showed no association between vitamin E
supplementation and cardiovascular events (nonfatal stroke or myocardial
infarction) or cardiovascular mortality. There is a long history of belief that topical application of vitamin E containing oil benefits burn and wound healing. This belief persists even though scientific reviews repeatedly refuted this claim.
The role of vitamin E in infant nutrition has a long research
history. From 1949 onward there were trials with premature infants
suggesting that oral alpha-tocopherol was protective against edema, intracranial hemorrhage, hemolytic anemia and retrolental fibroplasia.
A 2003 Cochrane review concluded that vitamin E supplementation in
preterm infants reduced the risk of intercranial hemorrhage and
retinopathy, but noted an increased risk of sepsis.
Research
As of
2018 there are at least 10 trials actively recruiting subjects for
conditions including liver disease, burn injury, skin aging, and type 2
diabetes.
Older listings of trials, some published, had as topics exercise,
infection, preventing atherosclerosis, burn injury, retinopathy in
premature infants, male infertility and type 2 diabetes.
Observational studies that measure dietary intake and/or serum concentration, and experimental studies that ideally are randomized clinical trials (RCTs), are two means of examining the effects or lack thereof of a proposed intervention on human health.
Healthcare outcomes are expected to be in accord between reviews of
observational and experimental studies. If, however, there is a lack of
agreement, then factors other than study design need to be considered.
For the conditions described below, the results of RCTs do not
always concur with the observational evidence. This could be a matter of
amount. Observational studies compare low consumers to high consumers
based on intake from food, whereas RCTS often used amounts of
alpha-tocopherol 20X to 30X higher than what can be achieved from food.
Diets higher in vitamin E may contain other compounds that convey health
benefits, so the observed effect may not be due to the vitamin E
content. There is also a concern that supplementing with
alpha-tocopherol in multiples much higher than is possible via diet will
suppress absorption and retention of other tocopherols, with unknown
effects on health. Supplementing alpha-tocopherol is known to reduced
serum gamma- and delta-tocopherol concentrations.
From one large survey, consumption of alpha-tocopherol as a supplement
lowered serum gamma-tocopherol from 6.0 micromol/L for people not
consuming any supplement to 2.1 micromol/L for those consuming greater
than or equal to 400 IU/day.
A Cochrane review published in 2017 on antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration
(AMD) identified only one vitamin E clinical trial. That trial compared
500 IU/day of alpha-tocopherol to placebo for four years and reported
no effect on the progression of AMD in people already diagnosed with the
condition.
Another Cochrane review, same year, same authors, reviewed the
literature on alpha-tocopherol preventing the development of AMD. This
review identified four trials, duration 4–10 years, and reported no
change to risk of developing AMD. A large clinical trial known as AREDS compared beta-carotene (15 mg), vitamin C
(500 mg) and alpha-tocopherol (400 IU) to placebo for up to 10 years,
with a conclusion that the anti-oxidant combination significantly slowed
progression. However, because there was no group in the trial receiving
only vitamin E, no conclusions could be drawn as to the contribution of
the vitamin to the effect.
Alzheimer's disease
Alzheimer's disease (AD) and vascular dementia
are common causes of decline of brain functions that occur with age. AD
is a chronic neurodegenerative disease that worsens over time. The disease process is associated with plaques and tangles in the brain. Vascular dementia can be caused by ischemic or hemorrhagic infarcts affecting multiple brain areas, including the anterior cerebral artery territory, the parietal lobes, or the cingulate gyrus.
Both types of dementia may be present. Vitamin E status (and that of
other antioxidant nutrients) is conjectured as having a possible impact
on risk of Alzheimer's disease and vascular dementia. A review of
dietary intake studies reported that higher consumption of vitamin E
from foods lowered the risk of developing AD by 24%.
A second review examined serum vitamin E levels and reported lower
serum vitamin E in AD patients compared to healthy, age-matched people.
A Cochrane review reported on vitamin E as treatment for mild cognitive
impairment (MCI) and Alzheimer's disease. Based on evidence from only
one trial in each of the categories, the authors' conclusions were that
there was not sufficient evidence for supplemental vitamin E preventing
the progression from MCI to dementia, but that it did slow functional
decline in people with AD. Given the small number of trials and
subjects, the authors recommended further research.
In 2017 a consensus statement from the British Association for
Psychopharmacology included that until further information is available,
vitamin E cannot be recommended for treatment or prevention of
Alzheimer's disease.
Cancer
An inverse relationship between dietary vitamin E and kidney cancer risk was reported in a meta-analysis
of observational studies. The relative risk reduction was 19% when
highest and lowest intake groups were compared. The authors concluded
that randomized controlled trials (RCTs) are needed. An inverse relationship between dietary vitamin E and bladder cancer
was reported in a meta-analysis of observational studies. The relative
risk reduction was 18% when highest and lowest intake groups were
compared. The authors concluded that large prospective studies are
needed to confirm this association.
A very large multi-year comparing placebo to an all
rac-alpha-tocopherol group consuming 400 IU/day reported no
statistically significant difference in bladder cancer cases. An inverse relationship between dietary vitamin E and lung cancer
risk was reported in a meta-analysis of observational studies. The
relative risk reduction was 16% when highest and lowest intake groups
were compared. The benefit was progressive as dietary intake increased
from 2 mg/day to 16 mg/day. The authors noted that the findings needs to
be confirmed by prospective studies.
One such large trial, which compared 50 mg alpha-tocopherol to placebo
in male tobacco smokers, reported no impact on lung cancer.
A very large trial, which tracked people who chose to consume a vitamin
E dietary supplement, reported an increased risk of lung cancer for
those consuming more than 215 mg/day.
For prostate cancer,
there are conflicting results. A meta-analysis based on serum
alpha-tocopherol content reported an inverse correlation, with the
difference between lowest and highest a 21% reduction in relative risk. In contrast, a meta-analysis of observational studies reported no relationship for dietary vitamin E intake.
There were also conflicting results from large RCTs. The ATBC trial
administered placebo or 50 mg/day alpha-tocopherol to male tobacco
smokers for 5 to 8 years and reported a 32% decrease in the incidence of
prostate cancer.
Conversely, the SELECT trial of selenium and vitamin E for prostate
cancer enrolled men ages 55 or older, mostly non-smokers, to consume a
placebo or a 400 IU/day dietary supplement. It reported relative risk as
a statistically significant 17% higher for the vitamin group.
For colorectal cancer,
a systematic review identified RCTs of vitamin E and placebo followed
for 7–10 years. There was a non-significant 11% decrease in relative
risk.
The SELECT trial (men over 55 years, placebo or 400 IU/day) also
reported on colorectal cancer. There was a non-significant 3% increase
in adenoma occurrence compared to placebo.
The Women's Health Study compared placebo to 600 IU of natural-source
vitamin E on alternate days for an average of 10.1 years. There were no
significant differences for incidences of all types of cancer, cancer
deaths, or for breast, lung or colon cancers.
Potential confounding factors are the form of vitamin E used in
prospective studies and the amounts. Synthetic, racemic mixtures of
vitamin E isomers are not bioequivalent to natural, non-racemic
mixtures, yet are widely used in clinical trials and as dietary
supplement ingredients.
One review reported a modest increase in cancer risk with vitamin E
supplementation while stating that more than 90% of the cited clinical
trials used the synthetic, racemic form dl-alpha-tocopherol.
Cancer health claims
The
U.S.A Food and Drug Administration initiated a process of reviewing and
approving food and dietary supplement health claims in 1993. Reviews of
petitions results in proposed claims being rejected or approved. If
approved, specific wording is allowed on package labels. In 1999 a
second process for claims review was created. If there is not a
scientific consensus on the totality of the evidence, a Qualified Health
Claim (QHC) may be established. The FDA does not “approve” qualified
health claim petitions. Instead, it issues a Letter of Enforcement
Discretion that includes very specific claim language and the
restrictions on using that wording.
The first QHCs relevant to vitamin E were issued in 2003: “Some
scientific evidence suggests that consumption of antioxidant vitamins
may reduce the risk of certain forms of cancer.” In 2009 the claims
became more specific, allowing that vitamin E might reduce the risk of
renal, bladder and colorectal cancers, but with required mention that
the evidence was deemed weak and the claimed benefits highly unlikely. A
petition to add brain, cervical, gastric and lung cancers was rejected.
A further revision, May 2012, allowed that vitamin E may reduce risk of
renal, bladder and colorectal cancers, with a more concise qualifier
sentence added: “FDA has concluded that there is very little scientific
evidence for this claim.” Any company product label making the cancer
claims has to include a qualifier sentence. The European Food Safety Authority (EFSA) reviews proposed health claims for the European Union countries. As of March 2018, EFSA has not evaluated any vitamin E and cancer prevention claims.
Cataracts
A
meta-analysis from 2015 reported that for studies which reported serum
tocopherol, higher serum concentration was associated with a 23%
reduction in relative risk of age-related cataracts
(ARC), with the effect due to differences in nuclear cataract rather
than cortical or posterior subcapsular cataract - the three major
classifications of age-related cataracts.
However, this article and a second meta-analysis reporting on clinical
trials of alpha-tocopherol supplementation reported no statistically
significant change to risk of ARC when compared to placebo.
Cardiovascular diseases
Research on the effects of vitamin E on cardiovascular disease has produced conflicting results. In theory, oxidative modification of LDL-cholesterol promotes blockages in coronary arteries that lead to atherosclerosis and heart attacks,
so vitamin E functioning as an antioxidant would reduce oxidized
cholesterol and lower risk of cardiovascular disease. Vitamin E status
has also been implicated in the maintenance of normal endothelial cell
function of cells lining the inner surface of arteries,
anti-inflammatory activity and inhibition of platelet adhesion and aggregation. An inverse relation has been observed between coronary heart disease and the consumption of foods high in vitamin E, and also higher serum concentration of alpha-tocopherol.
In one of the largest observational studies, almost 90,000 healthy
nurses were tracked for eight years. Compared to those in the lowest
fifth for reported vitamin E consumption (from food and dietary
supplements), those in the highest fifth were at a 34% lower risk of
major coronary disease.
The problem with observational studies is that these cannot confirm a
relation between the lower risk of coronary heart disease and vitamin E
consumption because of confounding factors. Diet higher in vitamin E may
also be higher in other, unidentified components that promote heart
health, or people choosing such diets may be making other healthy
lifestyle choices.
There is some supporting evidence from randomized clinical trials
(RCTs). A meta-analysis on the effects of alpha-tocopherol
supplementation in RCTs on aspects of cardiovascular health reported
that when consumed without any other antioxidant nutrient, the relative
risk of heart attack was reduced by 18%.
The results were not consistent for all of the individual trials
incorporated into the meta-analysis. For example, the Physicians' Health
Study II did not show any benefit after 400 IU every other day for
eight years, for heart attack, stroke, coronary mortality or all-cause
mortality.
The HOPE/HOPE-TOO trial, which enrolled people with pre-existing
vascular disease or diabetes into a multi-year trial of 400 IU/day,
reported a higher risk of heart failure in the alpha-tocopherol group.
The effects of vitamin E supplementation on incidence of stroke were summarized in 2011. There were no significant benefits for vitamin E versus placebo. Subset analysis for ischaemic stroke, haemorrhagic stroke,
fatal stroke, non-fatal stroke - all no significant difference in risk.
Likewise for subset analysis of natural or synthetic vitamin E, or only
above or below 300 IU/day, or whether the enrolled people were healthy
or considered to be at higher than normal risk. The authors concluded
that there was a lack of clinically important benefit of vitamin E
supplementation in the prevention of stroke.
One large, multi-year study in which post-menopausal women consumed
either placebo or 600 IU of natural-sourced vitamin E on alternate days
reported no effect on stroke, but did report a 21% reduction in relative risk of developing a deep vein clot or pulmonary embolism.
The beneficial effect was strongest is the subset of women who had a
history of a prior thrombotic event or who were genetically coded for
clot risk (factor V Leiden or prothrombin mutation).
Cardiovascular health claims
In 2001 the US Food and Drug Administration rejected proposed health claims for vitamin E and cardiovascular health.
The US National Institutes of Health reviewed literature published up
to 2008 and concluded "In general, clinical trials have not provided
evidence that routine use of vitamin E supplements prevents
cardiovascular disease or reduces its morbidity and mortality." The European Food Safety Authority (EFSA) reviews proposed health claims for the European Union
countries. In 2010 the EFSA reviewed and rejected claims that a cause
and effect relationship has been established between the dietary intake
of vitamin E and maintenance of normal cardiac function or of normal
blood circulation.
Nonalcoholic fatty liver disease
alpha-Tocopherol can be used in the treatment of nonalcoholic fatty liver disease (NAFLD) and the more extreme subset known as nonalcoholic steatohepatitis
(NASH). A meta-analysis reported that in controlled trials, vitamin E
significantly reduced elevated liver enzymes, steatosis, inflammation
and fibrosis.
Parkinson's disease
There
is an observed inverse correlation seen with dietary vitamin E, but no
confirming evidence from placebo-controlled clinical trials. A
meta-analysis published in 2005 concluded that diets higher in vitamin E
content lowered risk of developing Parkinson's disease.
From what appears to be the only clinical trial of tocopherol
supplementation in people with early Parkinson's disease, 2000 IU/day
for 14 months had no effect on rate of disease progression.
Pregnancy
Antioxidant
vitamins as dietary supplements have been proposed as having benefits
if consumed during pregnancy. For the combination of vitamin E with
vitamin C supplemented to pregnant women, a Cochrane review concluded
that the data do not support vitamin E supplementation - majority of
trials alpha-tocopherol at 400 IU/day plus vitamin C at 1000 mg/day - as
being efficacious for reducing risk of stillbirth, neonatal death, preterm birth, preeclampsia or any other maternal or infant outcomes, either in healthy women or those considered at risk for pregnancy complications.
The review identified only three small trials in which vitamin E was
supplemented without co-supplementation with vitamin C. None of these
trials reported any clinically meaningful information.
Topical
Although there is widespread use of tocopheryl acetate as a topical medication, with claims for improved wound healing and reduced scar tissue, reviews have repeatedly concluded that there is insufficient evidence to support these claims.
There are reports of vitamin E-induced allergic contact dermatitis from
use of vitamin-E derivatives such as tocopheryl linoleate and
tocopherol acetate in skin care products. Incidence is low despite
widespread use.