| Diphtheria | |
|---|---|
 | |
| Diphtheria can cause a swollen neck, sometimes referred to as a bull neck. | |
| Specialty | Infectious disease |
| Symptoms | Sore throat, fever, barky cough |
| Usual onset | 2–5 days post-exposure |
| Causes | Corynebacterium diphtheriae (spread by direct contact and through the air) |
| Diagnostic method | Throat appearance, culture |
| Prevention | Diphtheria vaccine |
| Treatment | Antibiotics, tracheostomy |
| Frequency | 4,500 (reported 2015) |
| Deaths | 2,100 (2015) |
Diphtheria is an infection caused by the bacterium Corynebacterium diphtheriae. Signs and symptoms may vary from mild to severe. They usually start two to five days after exposure. Symptoms often come on fairly gradually, beginning with a sore throat and fever. In severe cases, a grey or white patch develops in the throat. This can block the airway and create a barking cough as in croup. The neck may swell in part due to enlarged lymph nodes. A form of diphtheria which involves the skin, eyes or genitals also exists. Complications may include myocarditis, inflammation of nerves, kidney problems, and bleeding problems due to low levels of platelets. Myocarditis may result in an abnormal heart rate and inflammation of the nerves may result in paralysis.
Diphtheria is usually spread between people by direct contact or through the air. It may also be spread by contaminated objects. Some people carry the bacterium without having symptoms, but can still spread the disease to others. The three main types of C. diphtheriae cause different severities of disease. The symptoms are due to a toxin produced by the bacterium. Diagnosis can often be made based on the appearance of the throat with confirmation by microbiological culture. Previous infection may not protect against future infection.
A diphtheria vaccine is effective for prevention and available in a number of formulations. Three or four doses, given along with tetanus vaccine and pertussis vaccine, are recommended during childhood. Further doses of diphtheria-tetanus vaccine are recommended every ten years. Protection can be verified by measuring the antitoxin level in the blood. Diphtheria can be prevented in those exposed as well as treated with the antibiotics erythromycin or benzylpenicillin. A tracheotomy is sometimes needed to open the airway in severe cases.
In 2015, 4,500 cases were officially reported worldwide, down from nearly 100,000 in 1980. About a million cases a year are believed to have occurred before the 1980s. Diphtheria currently occurs most often in sub-Saharan Africa, India, and Indonesia. In 2015, it resulted in 2,100 deaths, down from 8,000 deaths in 1990. In areas where it is still common, children are most affected. It is rare in the developed world due to widespread vaccination but can re-emerge if vaccination rates decrease. In the United States, 57 cases were reported between 1980 and 2004. Death occurs in 5% to 10% of those diagnosed. The disease was first described in the 5th century BC by Hippocrates. The bacterium was identified in 1882 by Edwin Klebs.
Signs and symptoms
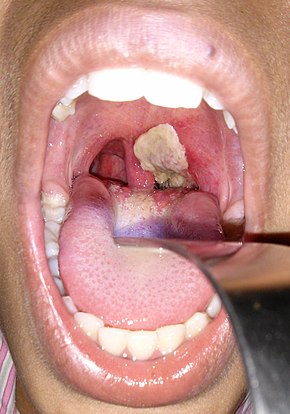
An adherent, dense, grey pseudomembrane covering the tonsils is classically seen in diphtheria.
A diphtheria skin lesion on the leg
The symptoms of diphtheria usually begin two to seven days after
infection. Symptoms of diphtheria include fever of 38 °C (100.4 °F) or
above; chills; fatigue; bluish skin coloration (cyanosis); sore throat; hoarseness; cough; headache; difficulty swallowing; painful swallowing; difficulty breathing; rapid breathing; foul-smelling and bloodstained nasal discharge; and lymphadenopathy.
Within two to three days, diphtheria may destroy healthy tissues in the
respiratory system. The dead tissue forms a thick, gray coating that
can build up in the throat or nose. This thick gray coating is called a
"pseudomembrane". It can cover tissues in the nose, tonsils, voice box,
and throat, making it very hard to breathe and swallow. Symptoms can also include cardiac arrhythmias, myocarditis, and cranial and peripheral nerve palsies.
Diphtheritic croup
Laryngeal
diphtheria can lead to a characteristic swollen neck and throat, or
"bull neck". The swollen throat is often accompanied by a serious
respiratory condition, characterized by a brassy or "barking" cough, stridor, hoarseness, and difficulty breathing; and historically referred to variously as "diphtheritic croup", "true croup", or sometimes simply as "croup". Diphtheritic croup is extremely rare in countries where diphtheria vaccination is customary. As a result, the term "" nowadays most often refers to an unrelated viral illness that produces similar but milder respiratory symptoms.
Transmission
Human-to-human
transmission of diphtheria typically occurs through the air when an
infected individual coughs or sneezes. Breathing in particles released
from the infected individual leads to infection. Contact with any lesions on the skin can also lead to transmission of diphtheria, but this is uncommon.
Indirect infections can occur, as well. If an infected individual
touches a surface or object, the bacteria can be left behind and remain
viable. Also, some evidence indicates diphtheria has the potential to be
zoonotic, but this has yet to be confirmed. Corynebacterium ulcerans has been found in some animals, which would suggest zoonotic potential.
Mechanism
Diphtheria toxin is produced by C. diphtheriae only when infected with a bacteriophage that integrates the toxin-encoding genetic elements into the bacteria.
Diphtheria toxin is a single, 60-kDa-molecular weight protein composed of two peptide chains, fragment A and fragment B, held together by a disulfide bond. Fragment B is a recognition subunit that gains the toxin entry into the host cell by binding to the EGF-like domain of heparin-binding EGF-like growth factor on the cell surface. This signals the cell to internalize the toxin within an endosome via receptor-mediated endocytosis. Inside the endosome, the toxin is split by a trypsin-like protease
into its individual A and B fragments. The acidity of the endosome
causes fragment B to create pores in the endosome membrane, thereby
catalysing the release of fragment A into the cell's cytoplasm.
Fragment A inhibits the synthesis of new proteins in the affected cell by catalyzing ADP-ribosylation of elongation factor EF-2—a protein that is essential to the translation step of protein synthesis. This ADP-ribosylation involves the transfer of an ADP-ribose from NAD+ to a diphthamide (a modified histidine) residue within the EF-2 protein. Since EF-2 is needed for the moving of tRNA from the A-site to the P-site of the ribosome during protein translation, ADP-ribosylation of EF-2 prevents protein synthesis.
ADP-ribosylation of EF-2 is reversed by giving high doses of nicotinamide (a form of vitamin B3), since this is one of the reaction's end products, and high amounts drive the reaction in the opposite direction.
Diagnosis
The current clinical case definition of diphtheria used by the United States' Centers for Disease Control and Prevention is based on both laboratory and clinical criteria.
Laboratory criteria
- Isolation of C. diphtheriae from a Gram stain or throat culture from a clinical specimen
- Histopathologic diagnosis of diphtheria by Albert's stain
Toxin demonstration
- In vivo tests (guinea pig inoculation): Subcutaneous and intracutaneous tests
- In vitro test: Elek's gel precipitation test, detection of tox gene by PCR, ELISA, ICA
Clinical criteria
- Upper respiratory tract illness with sore throat
- Low-grade fever (above 39 °C (102 °F) is rare)
- An adherent, dense, grey pseudomembrane covering the posterior aspect of the pharynx: in severe cases, it can extend to cover the entire tracheobronchial tree.
Case classification
- Probable: a clinically compatible case that is not laboratory-confirmed and is not epidemiologically linked to a laboratory-confirmed case
- Confirmed: a clinically compatible case that is either laboratory-confirmed or epidemiologically linked to a laboratory-confirmed case
Empirical treatment should generally be started in a patient in whom suspicion of diphtheria is high.
Prevention
Quinvaxem is a widely administered pentavalent vaccine, which is a combination of five vaccines in one that protect babies from diphtheria, among other common childhood diseases.
Diphtheria vaccine is usually combined at least with tetanus vaccine
(Td) and often with pertussis (DTP, DTaP, TdaP, Tdap) vaccines, as well.
Treatment
The disease may remain manageable, but in more severe cases, lymph nodes
in the neck may swell, and breathing and swallowing are more difficult.
People in this stage should seek immediate medical attention, as
obstruction in the throat may require intubation or a tracheotomy. Abnormal cardiac rhythms can occur early in the course of the illness or weeks later, and can lead to heart failure.
Diphtheria can also cause paralysis in the eye, neck, throat, or
respiratory muscles. Patients with severe cases are put in a hospital intensive care unit and given a diphtheria antitoxin (consisting of antibodies isolated from the serum of horses that have been challenged with diphtheria toxin).
Since antitoxin does not neutralize toxin that is already bound to
tissues, delaying its administration increases risk of death. Therefore,
the decision to administer diphtheria antitoxin is based on clinical
diagnosis, and should not await laboratory confirmation.
Antibiotics have not been demonstrated to affect healing of local
infection in diphtheria patients treated with antitoxin. Antibiotics
are used in patients or carriers to eradicate C. diphtheriae and prevent its transmission to others. The Centers for Disease Control and Prevention recommends either:
- Metronidazole
- Erythromycin is given (orally or by injection) for 14 days (40 mg/kg per day with a maximum of 2 g/d), or
- Procaine penicillin G is given intramuscularly for 14 days (300,000 U/d for patients weighing <10 600="" and="" d="" for="" kg="" nbsp="" those="" u="" weighing="">10 kg); patients with allergies to penicillin G or erythromycin can use rifampin or clindamycin.
In cases that progress beyond a throat infection, diphtheria toxin
spreads through the blood and can lead to potentially life-threatening
complications that affect other organs, such as the heart and kidneys.
Damage to the heart caused by the toxin affects the heart's ability to
pump blood or the kidneys' ability to clear wastes. It can also cause
nerve damage, eventually leading to paralysis. About 40% to 50% of those
left untreated can die.
Epidemiology
Disability-adjusted life year for diphtheria per 100,000 inhabitants in 2004
no data
≤ 1
1–2
2–3
3–4
4–5
5–6
6–7
7–9
9–10
10–15
15–50
≥ 50
Diphtheria cases reported to the World Health Organization between 1997 and 2006:
no data
1–49 reported cases
Between 50 and 99 reported cases
Over 100 reported cases
Diphtheria is fatal in between 5% and 10% of cases. In children under
five years and adults over 40 years, the fatality rate may be as much
as 20%. In 2013, it resulted in 3,300 deaths, down from 8,000 deaths in 1990.
The number of cases has changed over the course of the last 2 decades,
specifically throughout developing countries. Better standards of
living, mass immunization, improved diagnosis, prompt treatment, and
more effective health care have led to the decrease in cases worldwide.
However, although outbreaks are rare, they still occur worldwide,
especially in developed nations such as Germany among unvaccinated
children.
In Nazi Germany contagious diseases such as diphtheria were among the
leading causes of morbidity; they increased "after the mid-1920s,
doubled again between 1932 and 1937, and reached extremely high levels
during the war only to decline rapidly thereafter".
After the breakup of the former Soviet Union in the early 1990s,
vaccination rates in its constituent countries fell so low that an
explosion of diphtheria cases occurred. In 1991, 2,000 cases of
diphtheria occurred in the USSR. Between 1991 and 1998 as many as
200,000 cases in the Commonwealth of Independent States were reported, with 5,000 deaths.
History
In 1613, Spain experienced an epidemic of diphtheria. The year is known as El Año de los Garrotillos (The Year of Strangulations) in the history of Spain.
In 1735, a diphtheria epidemic swept through New England.
Before 1826, diphtheria was known by different names across the
world. In England, it was known as Boulogne sore throat, as it spread
from France. In 1826, Pierre Bretonneau gave the disease the name diphthérite (from Greek diphthera "leather") describing the appearance of pseudomembrane in the throat.
In 1856, Victor Fourgeaud described an epidemic of diphtheria in California.
In 1878, Queen Victoria's daughter Princess Alice and her family became infected with diphtheria, causing two deaths, Princess Marie of Hesse and by Rhine and Princess Alice herself.
In 1883, Edwin Klebs identified the bacterium causing diphtheria and named it Klebs-Loeffler bacterium. The club shape of this bacterium helped Edwin to differentiate it from other bacteria. Over the period of time, it was called Microsporon diphtheriticum, Bacillus diphtheriae, and Mycobacterium diphtheriae. Current nomenclature is Corynebacterium diphtheriae.
Friedrich Loeffler was the first person to cultivate C. diphtheriae in 1884. He used Koch's postulates to prove association between C. diphtheriae and diphtheria. He also showed that the bacillus produces an exotoxin.
A diphtheria immunisation scheme in London, 1941
Joseph P. O’Dwyer introduced the O'Dwyer tube for laryngeal intubation in patients with an obstructed larynx in 1885. It soon replaced tracheostomy as the emergency diphtheric intubation method.
In 1888, Emile Roux and Alexandre Yersin showed that a substance produced by C. diphtheriae caused symptoms of diphtheria in animals.
In 1890, Shibasaburo Kitasato and Emil von Behring immunized guinea pigs with heat-treated diphtheria toxin. They also immunized goats and horses in the same way and showed that an "antitoxin" made from serum of immunized animals could cure the disease in non-immunized animals. Behring used this antitoxin (now known to consist of antibodies that neutralize the toxin produced by C. diphtheriae)
for human trials in 1891, but they were unsuccessful. Successful
treatment of human patients with horse-derived antitoxin began in 1894,
after production and quantification of antitoxin had been optimized. Von Behring won the first Nobel Prize in medicine in 1901 for his work on diphtheria.
In 1895, H. K. Mulford Company of Philadelphia started production and testing of diphtheria antitoxin in the United States. Park and Biggs described the method for producing serum from horses for use in diphtheria treatment.
In 1897, Paul Ehrlich
developed a standardized unit of measure for diphtheria antitoxin. This
was the first ever standardization of a biological product, and played
an important role in future developmental work on sera and vaccines.
In 1901, 10 of 11 inoculated St. Louis children died from
contaminated diphtheria antitoxin. The horse from which the antitoxin
was derived died of tetanus. This incident, coupled with a tetanus outbreak in Camden, New Jersey, played an important part in initiating federal regulation of biologic products.
On 7 January 1904, Ruth Cleveland died of diphtheria at the age of 12 years in Princeton, New Jersey. Ruth was the eldest daughter of former President Grover Cleveland and the former first lady Frances Folsom.
In 1905, Franklin Royer, from Philadelphia's Municipal Hospital,
published a paper urging timely treatment for diphtheria and adequate
doses of antitoxin. In 1906, Clemens Pirquet and Béla Schick described serum sickness in children receiving large quantities of horse-derived antitoxin.
Between 1910 and 1911, Béla Schick developed the Schick test
to detect pre-existing immunity to diphtheria in an exposed person.
Only those who were not exposed to diphtheria were preferably
vaccinated. A massive, five-year campaign was coordinated by Dr. Schick.
As a part of the campaign, 85 million pieces of literature were
distributed by the Metropolitan Life Insurance Company
with an appeal to parents to "Save your child from diphtheria." A
vaccine was developed in the next decade, and deaths began declining
significantly in 1924.
A poster from the United Kingdom advertising diphtheria immunisation (published prior to 1962)
In 1919, in Dallas, Texas, 10 children were killed and 60 others made
seriously ill by toxic antitoxin which had passed the tests of the New
York State Health Department. Mulford Company of Philadelphia (manufacturers) paid damages in every case.
In the 1920s, each year an estimated 100,000 to 200,000
diphtheria cases and 13,000 to 15,000 deaths occurred in the United
States. Children represented a large majority of these cases and fatalities. One of the most infamous outbreaks of diphtheria was in Nome, Alaska; the "Great Race of Mercy" to deliver diphtheria antitoxin is now celebrated by the Iditarod Trail Sled Dog Race.
In 1926, Alexander Thomas Glenny increased the effectiveness of diphtheria toxoid (a modified version of the toxin used for vaccination) by treating it with aluminum salts. Vaccination with toxoid was not widely used until the early 1930s.
In 1943, diphtheria outbreaks accompanied war and disruption in Europe. The 1 million cases in Europe resulted in 50,000 deaths.
In 1949, 68 of 606 children died after diphtheria immunization due to improper manufacture of aluminum phosphate toxoid.
In 1974, the World Health Organization included DPT vaccine in their Expanded Programme on Immunization for developing countries.
In 1975, an outbreak of cutaneous diphtheria in Seattle, Washington, was reported.
In 1994, the Russian Federation had 39,703 diphtheria cases. By contrast, in 1990, only 1,211 cases were reported. Between 1990 and 1998, diphtheria caused 5000 deaths in the countries of the former Soviet Union.
In early May 2010, a case of diphtheria was diagnosed in Port-au-Prince, Haiti, after the devastating 2010 Haiti earthquake. The 15-year-old male patient died while workers searched for antitoxin.
In 2013, three children died of diphtheria in Hyderabad, India.
In early June 2015, a case of diphtheria was diagnosed at Vall d'Hebron University Hospital in Barcelona, Spain. The 6-year-old child who died of the illness had not been previously vaccinated due to parental opposition to vaccination. It was the first case of diphtheria in the country since 1986 as reported by "El Mundo" or from 1998, as reported by WHO.
In June 2016, a 3-year-old, 5-year-old, and 7-year-old girl died of diphtheria in Kedah, Malacca and Sabah, Malaysia.
In January 2017, more than 300 cases were recorded in Venezuela.
In November and December 2017, an outbreak of diphtheria occurred in Indonesia with more than 600 cases found and 38 fatalities.