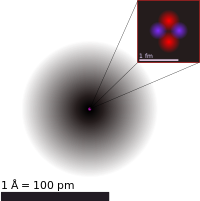
Atomic theory is the scientific theory that matter is composed of particles called atoms. Atomic theory traces its origins to an ancient philosophical tradition known as atomism. According to this idea, if one were to take a lump of matter and cut it into ever smaller pieces, one would reach a point where the pieces could not be further cut into anything smaller. Ancient Greek philosophers called these hypothetical ultimate particles of matter atomos, a word which meant "uncut".
In the early 1800s, the scientist John Dalton noticed that chemical substances seemed to combine and break down into other substances by weight in proportions that indicated that matter is indeed made of atoms as the ancient philosophers suspected. Shortly after 1850, certain physicists developed the kinetic theory of gases and of heat, which mathematically modelled the behavior of gases by assuming that gases are made of particles. In the early 20th century, Albert Einstein and Jean Perrin proved that Brownian motion (the erratic motion of pollen grains in water) is caused by the action of water molecules — this third line of evidence silenced remaining doubts among scientists as to whether atoms and molecules are real. Throughout the nineteenth century, some scientists had cautioned that the evidence for atoms was indirect, and therefore atoms might not actually be real, but only seem to be real.
By the early 20th century, scientists had developed fairly detailed and precise models for the structure of matter, which led to more rigorously-defined classifications for the tiny invisible particles that make up ordinary matter. An atom is now defined as the basic particle that composes a chemical element. Around the turn of the 20th century, physicists discovered that the particles that chemists called "atoms" are in fact agglomerations of even smaller particles (subatomic particles), but scientists kept the name out of convention. The term elementary particle is now used to refer to particles that are actually indivisible.
History
Philosophical atomism
The idea that matter is made up of discrete units is a very old idea, appearing in many ancient cultures such as Greece and India. The word "atom" (Greek: ἄτομος; atomos), meaning "uncuttable", was coined by the Pre-Socratic Greek philosophers Leucippus and his pupil Democritus (c.460–c.370 BC).
Democritus taught that atoms were infinite in number, uncreated, and eternal, and that the qualities of an object result from the kind of atoms that compose it. Democritus's atomism was refined and elaborated by the later Greek philosopher Epicurus (341–270 BC), and by the Roman Epicurean poet Lucretius (c.99–c.55 BC). During the Early Middle Ages, atomism was mostly forgotten in western Europe.During the 12th century, atomism became known again in western Europe through references to it in the newly-rediscovered writings of Aristotle.
In the 14th century, the rediscovery of major works describing atomist teachings, including Lucretius's De rerum natura and Diogenes Laërtius's Lives and Opinions of Eminent Philosophers, led to increased scholarly attention on the subject. Nonetheless, because atomism was associated with the philosophy of Epicureanism, which contradicted orthodox Christian teachings, belief in atoms was not considered acceptable by most European philosophers. The French Catholic priest Pierre Gassendi (1592–1655) revived Epicurean atomism with modifications, arguing that atoms were created by God and, though extremely numerous, are not infinite and the first person who use term "molecule" to describe aggregate of atom. Gassendi's modified theory of atoms was popularized in France by the physician François Bernier (1620–1688) and in England by the natural philosopher Walter Charleton (1619–1707). The chemist Robert Boyle (1627–1691) and the physicist Isaac Newton (1642–1727) both defended atomism and, by the end of the 17th century, it had become accepted by portions of the scientific community.
John Dalton
Near the end of the 18th century, two laws about chemical reactions emerged without referring to the notion of an atomic theory. The first was the law of conservation of mass, closely associated with the work of Antoine Lavoisier, which states that the total mass in a chemical reaction remains constant (that is, the reactants have the same mass as the products). The second was the law of definite proportions. First established by the French chemist Joseph Proust in 1797 this law states that if a compound is broken down into its constituent chemical elements, then the masses of the constituents will always have the same proportions by weight, regardless of the quantity or source of the original substance.
John Dalton studied and expanded upon this previous work and defended a new idea, later known as the law of multiple proportions: if the same two elements can be combined to form a number of different compounds, then the ratios of the masses of the two elements in their various compounds will be represented by small whole numbers. This is a common pattern in chemical reactions that was observed by Dalton and other chemists at the time.
Example 1 — tin oxides: Dalton identified two oxides of tin. One is a grey powder in which for every 100 parts of tin there is 13.5 parts of oxygen. The other oxide is a white powder in which for every 100 parts of tin there is 27 parts of oxygen. 13.5 and 27 form a ratio of 1:2. These oxides are today known as tin(II) oxide (SnO) and tin(IV) oxide (SnO2) respectively.
Example 2 — iron oxides: Dalton identified two oxides of iron. One is a black powder in which for every 100 parts of iron there is about 28 parts of oxygen. The other is a red powder in which for every 100 parts of iron there is 42 parts of oxygen. 28 and 42 form a ratio of 2:3. These oxides are today known as iron(II) oxide (better known as wüstite) and iron(III) oxide (the major constituent of rust). Their formulas are FeO and Fe2O3 respectively.
Example 3 — nitrogen oxides: There are three oxides of nitrogen in which for every 140 g of nitrogen, there is 80 g, 160 g, and 320 g of oxygen respectively, which gives a ratio of 1:2:4. These are nitrous oxide (N2O), nitric oxide (NO), and nitrogen dioxide (NO2) respectively.
This recurring pattern suggested that chemicals do not react in any arbitrary quantity, but in multiples of some basic indivisible unit of mass.
In his writings, Dalton used the term "atom" to refer to the basic particle of any chemical substance, not strictly for elements as is the practice today. Dalton did not use the word "molecule"; instead, he used the terms "compound atom" and "elementary atom".
Dalton believed atomic theory could also explain why water absorbed different gases in different proportions—for example, he found that water absorbed carbon dioxide far better than it absorbed nitrogen. Dalton hypothesized this was due to the differences in mass and complexity of the gases' respective particles. Indeed, carbon dioxide molecules (CO2) are heavier and larger than nitrogen molecules (N2).
Dalton proposed that each chemical element is composed of atoms of a single, unique type, and though they cannot be altered or destroyed by chemical means, they can combine to form more complex structures (chemical compounds). This marked the first truly scientific theory of the atom, since Dalton reached his conclusions by experimentation and examination of the results in an empirical fashion.
In 1803 Dalton orally presented his first list of relative atomic weights for a number of substances. This paper was published in 1805, but he did not discuss there exactly how he obtained these figures. The method was first revealed in 1807 by his acquaintance Thomas Thomson, in the third edition of Thomson's textbook, A System of Chemistry. Finally, Dalton published a full account in his own textbook, A New System of Chemical Philosophy, 1808 and 1810.
Dalton estimated the atomic weights according to the mass ratios in which they combined, with the hydrogen atom taken as unity. However, Dalton did not conceive that with some elements atoms exist in molecules—e.g. pure oxygen exists as O2. He also mistakenly believed that the simplest compound between any two elements is always one atom of each (so he thought water was HO, not H2O). This, in addition to the crudity of his equipment, flawed his results. For instance, in 1803 he believed that oxygen atoms were 5.5 times heavier than hydrogen atoms, because in water he measured 5.5 grams of oxygen for every 1 gram of hydrogen and believed the formula for water was HO. Adopting better data, in 1806 he concluded that the atomic weight of oxygen must actually be 7 rather than 5.5, and he retained this weight for the rest of his life. Others at this time had already concluded that the oxygen atom must weigh 8 relative to hydrogen equals 1, if one assumes Dalton's formula for the water molecule (HO), or 16 if one assumes the modern water formula (H2O).
Avogadro
The flaw in Dalton's theory was corrected in principle in 1811 by Amedeo Avogadro. Avogadro had proposed that equal volumes of any two gases, at equal temperature and pressure, contain equal numbers of molecules (in other words, the mass of a gas's particles does not affect the volume that it occupies). Avogadro's law allowed him to deduce the diatomic nature of numerous gases by studying the volumes at which they reacted. For instance: since two liters of hydrogen will react with just one liter of oxygen to produce two liters of water vapor (at constant pressure and temperature), it meant a single oxygen molecule splits in two in order to form two particles of water. Thus, Avogadro was able to offer more accurate estimates of the atomic mass of oxygen and various other elements, and made a clear distinction between molecules and atoms.
Brownian Motion
In 1827, the British botanist Robert Brown observed that dust particles inside pollen grains floating in water constantly jiggled about for no apparent reason. In 1905, Albert Einstein theorized that this Brownian motion was caused by the water molecules continuously knocking the grains about, and developed a hypothetical mathematical model to describe it. This model was validated experimentally in 1908 by French physicist Jean Perrin, thus providing additional validation for particle theory (and by extension atomic theory).
Discovery of subatomic particles
Atoms were thought to be the smallest possible division of matter until 1897 when J. J. Thomson discovered the electron through his work on cathode rays.
A Crookes tube is a sealed glass container in which two electrodes are separated by a vacuum. When a voltage is applied across the electrodes, cathode rays are generated, creating a glowing patch where they strike the glass at the opposite end of the tube. Through experimentation, Thomson discovered that the rays could be deflected by an electric field (in addition to magnetic fields, which was already known). He concluded that these rays, rather than being a form of light, were composed of very light negatively charged particles he called "corpuscles" (they would later be renamed electrons by other scientists). He measured the mass-to-charge ratio and discovered it was 1800 times smaller than that of hydrogen, the smallest atom. These corpuscles were a particle unlike any other previously known.
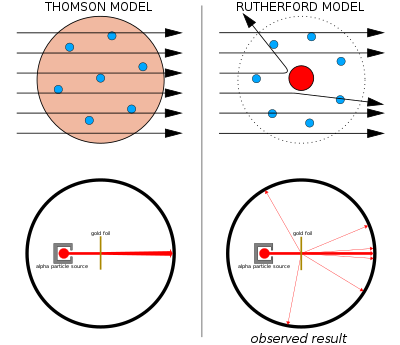
Thomson suggested that atoms were divisible, and that the corpuscles were their building blocks. To explain the overall neutral charge of the atom, he proposed that the corpuscles were distributed in a uniform sea of positive charge; this was the plum pudding model as the electrons were embedded in the positive charge like raisins in a plum pudding (although in Thomson's model they were not stationary).
Discovery of the nucleus
Left: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
Right: Observed results: a small portion of the particles were deflected by the concentrated positive charge of the nucleus.
Thomson's plum pudding model was disproved in 1909 by one of his former students, Ernest Rutherford, who discovered that most of the mass and positive charge of an atom is concentrated in a very small fraction of its volume, which he assumed to be at the very center.
Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden came to have doubts about the Thomson model after they encountered difficulties when they tried to build an instrument to measure the charge-to-mass ratio of alpha particles (these are positively-charged particles emitted by certain radioactive substances such as radium). The alpha particles were being scattered by the air in the detection chamber, which made the measurements unreliable. Thomson had encountered a similar problem in his work on cathode rays, which he solved by creating a near-perfect vacuum in his instruments. Rutherford didn't think he'd run into this same problem because alpha particles are much heavier than electrons. According to Thomson's model of the atom, the positive charge in the atom is not concentrated enough to produce an electric field strong enough to deflect an alpha particle, and the electrons are so lightweight they should be pushed aside effortlessly by the much heavier alpha particles. Yet there was scattering, so Rutherford and his colleagues decided to investigate this scattering carefully.
Between 1908 and 1913, Rutheford and his colleagues performed a series of experiments in which they bombarded thin foils of metal with alpha particles. They spotted alpha particles being deflected by angles greater than 90°. To explain this, Rutherford proposed that the positive charge of the atom is not distributed throughout the atom's volume as Thomson believed, but is concentrated in a tiny nucleus at the center. Only such an intense concentration of charge could produce an electric field strong enough to deflect the alpha particles as observed.
First steps toward a quantum physical model of the atom
The planetary model of the atom had two significant shortcomings. The first is that, unlike planets orbiting a sun, electrons are charged particles. An accelerating electric charge is known to emit electromagnetic waves according to the Larmor formula in classical electromagnetism. An orbiting charge should steadily lose energy and spiral toward the nucleus, colliding with it in a small fraction of a second. The second problem was that the planetary model could not explain the highly peaked emission and absorption spectra of atoms that were observed.
Quantum theory revolutionized physics at the beginning of the 20th century, when Max Planck and Albert Einstein postulated that light energy is emitted or absorbed in discrete amounts known as quanta (singular, quantum). In 1913, Niels Bohr incorporated this idea into his Bohr model of the atom, in which an electron could only orbit the nucleus in particular circular orbits with fixed angular momentum and energy, its distance from the nucleus (i.e., their radii) being proportional to its energy. Under this model an electron could not spiral into the nucleus because it could not lose energy in a continuous manner; instead, it could only make instantaneous "quantum leaps" between the fixed energy levels. When this occurred, light was emitted or absorbed at a frequency proportional to the change in energy (hence the absorption and emission of light in discrete spectra).
Bohr's model was not perfect. It could only predict the spectral lines of hydrogen; it couldn't predict those of multielectron atoms. Worse still, as spectrographic technology improved, additional spectral lines in hydrogen were observed which Bohr's model couldn't explain. In 1916, Arnold Sommerfeld added elliptical orbits to the Bohr model to explain the extra emission lines, but this made the model very difficult to use, and it still couldn't explain more complex atoms.
Discovery of isotopes
While experimenting with the products of radioactive decay, in 1913 radiochemist Frederick Soddy discovered that there appeared to be more than one element at each position on the periodic table. The term isotope was coined by Margaret Todd as a suitable name for these elements.
That same year, J. J. Thomson conducted an experiment in which he channeled a stream of neon ions through magnetic and electric fields, striking a photographic plate at the other end. He observed two glowing patches on the plate, which suggested two different deflection trajectories. Thomson concluded this was because some of the neon ions had a different mass. The nature of this differing mass would later be explained by the discovery of neutrons in 1932.
Discovery of nuclear particles
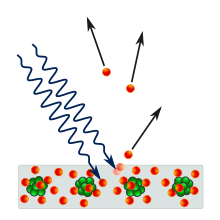
In 1917 Rutherford bombarded nitrogen gas with alpha particles and observed hydrogen nuclei being emitted from the gas (Rutherford recognized these, because he had previously obtained them bombarding hydrogen with alpha particles, and observing hydrogen nuclei in the products). Rutherford concluded that the hydrogen nuclei emerged from the nuclei of the nitrogen atoms themselves (in effect, he had split a nitrogen).
From his own work and the work of his students Bohr and Henry Moseley, Rutherford knew that the positive charge of any atom could always be equated to that of an integer number of hydrogen nuclei. This, coupled with the atomic mass of many elements being roughly equivalent to an integer number of hydrogen atoms - then assumed to be the lightest particles - led him to conclude that hydrogen nuclei were singular particles and a basic constituent of all atomic nuclei. He named such particles protons. Further experimentation by Rutherford found that the nuclear mass of most atoms exceeded that of the protons it possessed; he speculated that this surplus mass was composed of previously-unknown neutrally charged particles, which were tentatively dubbed "neutrons".
In 1928, Walter Bothe observed that beryllium emitted a highly penetrating, electrically neutral radiation when bombarded with alpha particles. It was later discovered that this radiation could knock hydrogen atoms out of paraffin wax. Initially it was thought to be high-energy gamma radiation, since gamma radiation had a similar effect on electrons in metals, but James Chadwick found that the ionization effect was too strong for it to be due to electromagnetic radiation, so long as energy and momentum were conserved in the interaction. In 1932, Chadwick exposed various elements, such as hydrogen and nitrogen, to the mysterious "beryllium radiation", and by measuring the energies of the recoiling charged particles, he deduced that the radiation was actually composed of electrically neutral particles which could not be massless like the gamma ray, but instead were required to have a mass similar to that of a proton. Chadwick now claimed these particles as Rutherford's neutrons. For his discovery of the neutron, Chadwick received the Nobel Prize in 1935.
Quantum physical models of the atom
In 1924, Louis de Broglie proposed that all moving particles—particularly subatomic particles such as electrons—exhibit a degree of wave-like behavior. Erwin Schrödinger, fascinated by this idea, explored whether or not the movement of an electron in an atom could be better explained as a wave rather than as a particle. Schrödinger's equation, published in 1926, describes an electron as a wave function instead of as a point particle. This approach elegantly predicted many of the spectral phenomena that Bohr's model failed to explain. Although this concept was mathematically convenient, it was difficult to visualize, and faced opposition. One of its critics, Max Born, proposed instead that Schrödinger's wave function described not the electron but rather all its possible states, and thus could be used to calculate the probability of finding an electron at any given location around the nucleus. This reconciled the two opposing theories of particle versus wave electrons and the idea of wave–particle duality was introduced. This theory stated that the electron may exhibit the properties of both a wave and a particle. For example, it can be refracted like a wave, and has mass like a particle.
A consequence of describing electrons as waveforms is that it is mathematically impossible to simultaneously derive the position and momentum of an electron. This became known as the Heisenberg uncertainty principle after the theoretical physicist Werner Heisenberg, who first described it and published it in 1927. This invalidated Bohr's model, with its neat, clearly defined circular orbits. The modern model of the atom describes the positions of electrons in an atom in terms of probabilities. An electron can potentially be found at any distance from the nucleus, but, depending on its energy level, exists more frequently in certain regions around the nucleus than others; this pattern is referred to as its atomic orbital. The orbitals come in a variety of shapes-sphere, dumbbell, torus, etc.-with the nucleus in the middle.





















![\psi (x,t)=[A\sin(kx)+B\cos(kx)]\mathrm {e} ^{-i\omega t},](https://wikimedia.org/api/rest_v1/media/math/render/svg/ad52c107f2d14f50309f183f39a3b73c2bdf2234)























































































