Proteins separated by SDS-PAGE, Coomassie Brilliant Blue staining
Protein electrophoresis is a method for analysing the proteins
in a fluid or an extract. The electrophoresis may be performed with a
small volume of sample in a number of alternative ways with or without a
supporting medium: SDS polyacrylamide gel electrophoresis (in short: gel electrophoresis, PAGE, or SDS-electrophoresis), free-flow electrophoresis, electrofocusing, isotachophoresis, affinity electrophoresis, immunoelectrophoresis, counterelectrophoresis, and capillary electrophoresis. Each method has many variations with individual advantages and limitations. Gel electrophoresis is often performed in combination with electroblotting immunoblotting
to give additional information about a specific protein. Because of
practical limitations, protein electrophoresis is generally not suited
as a preparative method.
Denaturing gel methods
SDS-PAGE
SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis, describes a collection of related techniques to separate proteins according to their electrophoretic mobility (a function of the molecular weight of a polypeptide chain) while in the denatured
(unfolded) state. In most proteins, the binding of SDS to the
polypeptide chain imparts an even distribution of charge per unit mass,
thereby resulting in a fractionation by approximate size during
electrophoresis.
SDS is a strong detergent agent used to denature native proteins to unfolded, individual polypeptides. When a protein mixture is heated to 100 °C in presence of SDS, the detergent
wraps around the polypeptide backbone. In this process, the intrinsic
charges of polypeptides becomes negligible when compared to the negative
charges contributed by SDS. Thus polypeptides after treatment become
rod-like structures possessing a uniform charge density, that is same
net negative charge per unit length. The electrophoretic mobilities of
these proteins will be a linear function of the logarithms of their molecular weights.
Native gel methods
Native
gels, also known as non-denaturing gels, analyze proteins that are
still in their folded state. Thus, the electrophoretic mobility depends
not only on the charge-to-mass ratio, but also on the physical shape
and size of the protein.
Blue native PAGE
BN-PAGE is a native PAGE technique, where the Coomassie Brilliant Blue dye provides the necessary charges to the protein complexes for the electrophoretic separation. The disadvantage of Coomassie is that in binding to proteins it can act like a detergent causing complexes to dissociate. Another drawback is the potential quenching of chemoluminescence (e.g. in subsequent western blot detection or activity assays) or fluorescence of proteins with prosthetic groups (e.g. heme or chlorophyll) or labelled with fluorescent dyes.
Clear native PAGE
CN-PAGE (commonly referred to as Native PAGE) separates acidic water-soluble and membrane proteins in a polyacrylamide
gradient gel. It uses no charged dye so the electrophoretic mobility of
proteins in CN-PAGE (in contrast to the charge shift technique BN-PAGE)
is related to the intrinsic charge of the proteins.
The migration distance depends on the protein charge, its size and the
pore size of the gel. In many cases this method has lower resolution
than BN-PAGE, but CN-PAGE offers advantages whenever Coomassie
dye would interfere with further analytical techniques, for example it
has been described as a very efficient microscale separation technique
for FRET analyses. Also CN-PAGE is milder than BN-PAGE so it can retain labile supramolecular assemblies of membrane protein complexes that are dissociated under the conditions of BN-PAGE.
Quantitative native PAGE
The folded protein complexes
of interest separate cleanly and predictably due to the specific
properties of the polyacrylamide gel. The separated proteins are
continuously eluted into a physiological eluent and transported to a
fraction collector. In four to five PAGE fractions each the metal
cofactors can be identified and absolutely quantified by high-resolution
ICP-MS. The respective structures of the isolated metalloproteins can be determined by solution NMR spectroscopy.
Buffer systems
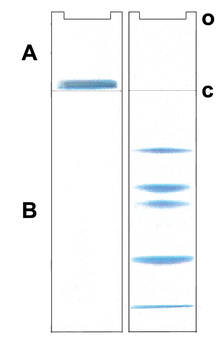
Postulated
migration of proteins in a Laemmli gel system A: Stacking gel, B:
Resolving gel, o: sample application c: discontinuities in the buffer
and electrophoretic matrix
Most protein separations are performed using a "discontinuous" (or DISC) buffer
system that significantly enhances the sharpness of the bands within
the gel. During electrophoresis in a discontinuous gel system, an ion
gradient is formed in the early stage of electrophoresis that causes all
of the proteins to focus into a single sharp band. The formation of the
ion gradient is achieved by choosing a pH value at which the ions of
the buffer are only moderately charged compared to the SDS-coated
proteins. These conditions provide an environment in which Kohlrausch's reactions determine the molar conductivity.
As a result, SDS-coated proteins are concentrated to several fold in a
thin zone of the order of 19 μm within a few minutes. At this stage all
proteins migrate at the same migration speed by isotachophoresis.
This occurs in a region of the gel that has larger pores so that the
gel matrix does not retard the migration during the focusing or
"stacking" event.
Separation of the proteins by size is achieved in the lower,
"resolving" region of the gel. The resolving gel typically has a much
smaller pore size, which leads to a sieving effect that now determines
the electrophoretic mobility of the proteins. At the same time, the
separating part of the gel also has a pH value in which the buffer ions
on average carry a greater charge, causing them to "outrun" the
SDS-covered proteins and eliminate the ion gradient and thereby the
stacking effect.
A very widespread discontinuous buffer system is the tris-glycine or "Laemmli" system that stacks at a pH of 6.8 and resolves at a pH of ~8.3-9.0. A drawback of this system is that these pH values may promote disulfide bond formation between cysteine residues in the proteins because the pKa
of cysteine ranges from 8-9 and because reducing agent present in the
loading buffer doesn't co-migrate with the proteins. Recent advances in
buffering technology alleviate this problem by resolving the proteins at
a pH well below the pKa of cysteine (e.g., bis-tris,
pH 6.5) and include reducing agents (e.g. sodium bisulfite) that move
into the gel ahead of the proteins to maintain a reducing environment.
An additional benefit of using buffers with lower pH values is that the
acrylamide gel is more stable at lower pH values, so the gels can be
stored for long periods of time before use.
SDS gradient gel electrophoresis of proteins
As
voltage is applied, the anions (and negatively charged sample
molecules) migrate toward the positive electrode (anode) in the lower
chamber, the leading ion is Cl−
( high mobility and high concentration); glycinate is the trailing ion
(low mobility and low concentration). SDS-protein particles do not
migrate freely at the border between the Cl− of the gel buffer and the Gly− of the cathode buffer. Friedrich Kohlrausch found that Ohm's law also applies to dissolved electrolytes. Because of the voltage drop between the Cl− and Glycine-buffers, proteins are compressed (stacked) into micrometer thin layers.
The boundary moves through a pore gradient and the protein stack
gradually disperses due to a frictional resistance increase of the gel
matrix. Stacking and unstacking occurs continuously in the gradient gel,
for every protein at a different position. For a complete protein
unstacking the polyacrylamide-gel concentration must exceed 16% T. The
two-gel system of "Laemmli" is a simple gradient gel. The pH
discontinuity of the buffers is of no significance for the separation
quality, and a "stacking-gel" with a different pH is not needed.
Visualization
The most popular protein stain is Coomassie Brilliant Blue.
It is an anionic dye, which non-specifically binds to proteins.
Proteins in the gel are fixed by acetic acid and simultaneously stained.
The excess dye incorporated into the gel can be removed by destaining
with the same solution without the dye. The proteins are detected as
blue bands on a clear background.
When more sensitive method than staining by Coomassie is needed
silver staining is usually used. Silver staining is a sensitive
procedure to detect trace amounts of proteins in gels, but can also
visualize nucleic acid or polysaccharides.
Visualization methods without using a dye such as Coomassie and silver are available on the market. For example Bio-Rad Laboratories markets ”stain-free” gels for SDS-PAGE gel electrophoresis.
Similarly as in nucleic acid gel electrophoresis, tracking dye
is often used. Anionic dyes of a known electrophoretic mobility are
usually included in the sample buffer. A very common tracking dye is Bromophenol blue.
This dye is coloured at alkali and neutral pH and is a small negatively
charged molecule that moves towards the anode. Being a highly mobile
molecule it moves ahead of most proteins.
Medical applications
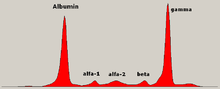
Schematic representation of a protein electrophoresis gel.
Serum protein electrophoresis showing a paraprotein (peak in the gamma zone) in a patient with multiple myeloma.
In medicine, protein electrophoresis is a method of analysing the proteins mainly in blood serum. Before the widespread use of gel electrophoresis, protein electrophoresis was performed as free-flow electrophoresis (on paper) or as immunoelectrophoresis.
Traditionally, two classes of blood proteins are considered: serum albumin and globulin. They are generally equal in proportion, but albumin
as a molecule is much smaller and lightly, negatively-charged, leading
to an accumulation of albumin on the electrophoretic gel. A small band
before albumin represents transthyretin
(also named prealbumin). Some forms of medication or body chemicals can
cause their own band, but it usually is small. Abnormal bands (spikes)
are seen in monoclonal gammopathy of undetermined significance and multiple myeloma, and are useful in the diagnosis of these conditions.
The globulins are classified by their banding pattern (with their main representatives):
- The alpha (α) band consists of two parts, 1 and 2:
- α1 - α1-antitrypsin, α1-acid glycoprotein.
- α2 - haptoglobin, α2-macroglobulin, α2-antiplasmin, ceruloplasmin.
- The beta (β) band - transferrin, LDL, complement
- The gamma (γ) band - immunoglobulin (IgA, IgD, IgE, IgG and IgM). Paraproteins (in multiple myeloma) usually appear in this band.
Normal present medical procedure involves determination of numerous
proteins in plasma including hormones and enzymes, some of them also
determined by electrophoresis. However, gel electrophoresis is mainly a
research tool, also when the subject is blood proteins.