Air-sea exchange of CO2
The biological pump, in its simplest form, is the ocean's biologically driven sequestration of carbon from the atmosphere to the ocean interior and seafloor sediments. It is the part of the oceanic carbon cycle responsible for the cycling of organic matter formed mainly by phytoplankton during photosynthesis (soft-tissue pump), as well as the cycling of calcium carbonate (CaCO3) formed into shells by certain organisms such as plankton and mollusks (carbonate pump).
Overview
The biological pump can be divided into three distinct phases, the first of which is the production of fixed carbon by planktonic phototrophs in the euphotic (sunlit) surface region of the ocean. In these surface waters, phytoplankton use carbon dioxide (CO2), nitrogen (N), phosphorus (P), and other trace elements (barium, iron, zinc, etc.) during photosynthesis to make carbohydrates, lipids, and proteins. Some plankton, (e.g. coccolithophores and foraminifera) combine calcium (Ca) and dissolved carbonates (carbonic acid and bicarbonate) to form a calcium carbonate (CaCO3) protective coating.
Once this carbon is fixed into soft or hard tissue, the organisms
either stay in the euphotic zone to be recycled as part of the
regenerative nutrient cycle
or once they die, continue to the second phase of the biological pump
and begin to sink to the ocean floor. The sinking particles will often
form aggregates as they sink, greatly increasing the sinking rate. It is
this aggregation that gives particles a better chance of escaping
predation and decomposition in the water column and eventually make it
to the sea floor.
The fixed carbon that is either decomposed by bacteria on the way
down or once on the sea floor then enters the final phase of the pump
and is remineralized to be used again in primary production.
The particles that escape these processes entirely are sequestered in
the sediment and may remain there for millions of years. It is this
sequestered carbon that is responsible for ultimately lowering
atmospheric CO2.
Primary production
The
first step in the biological pump is the synthesis of both organic and
inorganic carbon compounds by phytoplankton in the uppermost, sunlit
layers of the ocean. Organic compounds in the form of sugars, carbohydrates, lipids, and proteins are synthesized during the process of photosynthesis:
CO2 + H2O + light → CH2O + O2
In addition to carbon, organic matter found in phytoplankton is composed of nitrogen, phosphorus and various trace metals. The ratio of carbon to nitrogen and phosphorus varies little and has an average ratio of 106C:16N:1P, known as the Redfield ratio.
Trace metals such as magnesium, cadmium, iron, calcium, barium and
copper are orders of magnitude less prevalent in phytoplankton organic
material, but necessary for certain metabolic processes and therefore
can be limiting nutrients in photosynthesis due to their lower abundance
in the water column.
Oceanic primary production accounts for about half of the carbon
fixation carried out on Earth. Approximately 50–60 Pg of carbon are
fixed by marine phytoplankton each year despite the fact that they
comprise less than 1% of the total photosynthetic biomass on Earth. The
majority of this carbon fixation (~80%) is carried out in the open ocean
while the remaining amount occurs in the very productive upwelling
regions of the ocean. Despite these productive regions producing 2 to 3
times as much fixed carbon per area, the open ocean accounts for
greater than 90% of the ocean area and therefore is the larger
contributor.
Calcium carbonate
Carbon is also biologically fixed in the form of calcium carbonate (CaCO3)
used as a protective coating for many planktonic species
(coccolithophores, foraminifera) as well as larger marine organisms
(mollusk shells). While this form of carbon is not directly taken from
the atmospheric budget, it is formed from dissolved forms of carbonate
which are in equilibrium with CO2 and then responsible for removing this carbon via sequestration.
CO2 + H2O → H2CO3 → H+ + HCO3−
Ca2+ + 2HCO3− → CaCO3 + CO2 + H2O
While this process does manage to fix a large amount of carbon, two units of alkalinity are sequestered for every unit of sequestered carbon, thereby lowering the pH of surface water and raising atmospheric CO2. The formation and sinking of CaCO3 drives a surface to deep alkalinity gradient which serves to raise the partial pressure of dissolved CO2 in surface waters and actually raise atmospheric levels. In addition, the sequestration of CaCO3 serves to lower overall oceanic alkalinity and again raise atmospheric levels.
Marine snow
The
vast majority of carbon incorporated in organic and inorganic
biological matter is formed at the sea surface and then must sink to the
ocean floor. A single phytoplankton cell has a sinking rate around 1 m
per day and with 4000 m as the average depth of the ocean, it can take
over ten years for these cells to reach the ocean floor. However,
through processes such as coagulation and expulsion in predator fecal
pellets, these cells form aggregates. These aggregates, known as marine snow,
have sinking rates orders of magnitude greater than individual cells
and complete their journey to the deep in a matter of days.
White Cliffs of Dover
Of the 50–60 Pg of carbon fixed annually, roughly 10% leaves the
surface mixed layer of the oceans, while less than 0.5% of eventually
reaches the sea floor.
Most is retained in regenerated production in the euphotic zone and a
significant portion is remineralized in midwater processes during
particle sinking. The portion of carbon that leaves the surface mixed
layer of the ocean is sometimes considered "sequestered", and
essentially removed from contact with the atmosphere for many centuries. However, work also finds that, in regions such as the Southern Ocean, much of this carbon can quickly (within decades) come back into contact with the atmosphere.
The portion of carbon that makes it to the sea floor becomes part of
the geologic record and in the case of the calcium carbonate, may form
large deposits and resurface through tectonic motion as in the case with
the White Cliffs of Dover in Southern England. These cliffs are made almost entirely of the plates of buried coccolithophores.
Quantification
As
the biological pump plays an important role in the Earth's carbon
cycle, significant effort is spent quantifying its strength. However,
because they occur as a result of poorly constrained ecological
interactions usually at depth, the processes that form the biological
pump are difficult to measure. A common method is to estimate primary
production fuelled by nitrate and ammonium
as these nutrients have different sources that are related to the
remineralisation of sinking material. From these it is possible to
derive the so-called f-ratio,
a proxy for the local strength of the biological pump. Applying the
results of local studies to the global scale is complicated by the role
the ocean's circulation plays in different ocean regions.
The biological pump has a physico-chemical counterpart known as the solubility pump. For an overview of both pumps, see Raven & Falkowski (1999).
Anthropogenic changes
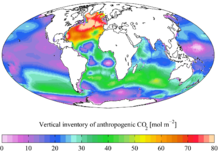
Estimated vertical inventory of "present day" (1990s) anthropogenic CO2
It was recently determined that coccolithophore concentrations in the North Atlantic have increased by an order of magnitude since the 1960s and an increase in absorbed CO2, as well as temperature, were modeled to be the most likely cause of this increase.
Changes in land use, the combustion of fossil fuels, and the production of cement have led to an increase in CO2 concentration in the atmosphere. At present, about one third (approximately 2 Pg C y−1 = 2 × 1015 grams of carbon per year) of anthropogenic emissions of CO2
are believed to be entering the ocean. However, the biological pump is
not believed to play a significant role in the net uptake of CO2
by oceans. This is because the biological pump is primarily limited by
the availability of light and nutrients, and not by carbon. This is in
contrast to the situation on land, where elevated atmospheric
concentrations of CO2 may increase primary production because land plants are able to improve their water-use efficiency (= decrease transpiration) when CO2 is easier to obtain.
However, there are still considerable uncertainties in the marine
carbon cycle, and some research suggests that a link between elevated CO2 and marine primary production exists.
However, climate change may affect the biological pump in the future by warming and stratifying
the surface ocean. It is believed that this could decrease the supply
of nutrients to the euphotic zone, reducing primary production there.
Also, changes in the ecological success of calcifying organisms caused
by ocean acidification may affect the biological pump by altering the strength of the hard tissues pump.
This may then have a "knock-on" effect on the soft tissues pump because
calcium carbonate acts to ballast sinking organic material.
In 2019, a study indicated that at current rates of seawater
acidification, we could see Antarctic phytoplanktons smaller and less
effective at storing carbon before the end of the century.