| Rabies | |
|---|---|
 | |
| A dog with rabies in the paralytic (post-furious) stage | |
| Specialty | Infectious disease |
| Symptoms | Fever, fear of water, confusion, excessive salivation, hallucinations, trouble sleeping, paralysis, coma |
| Causes | Rabies virus and Australian bat lyssavirus |
| Prevention | Rabies vaccine, animal control, rabies immunoglobulin |
| Prognosis | Nearly always death |
| Deaths | 17,400 (2015) |
Rabies is a viral disease that causes inflammation of the brain in humans and other mammals. Early symptoms can include fever and tingling at the site of exposure. These symptoms are followed by one or more of the following symptoms: violent movements, uncontrolled excitement, fear of water, an inability to move parts of the body, confusion, and loss of consciousness. Once symptoms appear, the result is nearly always death. The time period between contracting the disease and the start of symptoms is usually one to three months, but can vary from less than one week to more than one year. The time depends on the distance the virus must travel along peripheral nerves to reach the central nervous system.
Rabies is caused by lyssaviruses, including the rabies virus and Australian bat lyssavirus. It is spread when an infected animal scratches or bites another animal or human. Saliva from an infected animal can also transmit rabies if the saliva comes into contact with the eyes, mouth, or nose. Globally, dogs are the most common animal involved. More than 99% of rabies cases in countries where dogs commonly have the disease are the direct result of dog bites. In the Americas, bat bites are the most common source of rabies infections in humans, and less than 5% of cases are from dogs. Rodents are very rarely infected with rabies. The disease can be diagnosed only after the start of symptoms.
Animal control and vaccination programs have decreased the risk of rabies from dogs in a number of regions of the world. Immunizing people before they are exposed is recommended for those at high risk, including those who work with bats or who spend prolonged periods in areas of the world where rabies is common. In people who have been exposed to rabies, the rabies vaccine and sometimes rabies immunoglobulin are effective in preventing the disease if the person receives the treatment before the start of rabies symptoms. Washing bites and scratches for 15 minutes with soap and water, povidone-iodine, or detergent may reduce the number of viral particles and may be somewhat effective at preventing transmission. As of 2016, only fourteen people had survived a rabies infection after showing symptoms.
Rabies caused about 17,400 deaths worldwide in 2015. More than 95% of human deaths from rabies occur in Africa and Asia. About 40% of deaths occur in children under the age of 15. Rabies is present in more than 150 countries and on all continents but Antarctica. More than 3 billion people live in regions of the world where rabies occurs. A number of countries, including Australia and Japan, as well as much of Western Europe, do not have rabies among dogs. Many Pacific islands do not have rabies at all. It is classified as a neglected tropical disease.
Signs and symptoms
A person with rabies, 1959
The period between infection and the first symptoms (incubation period) is typically 1–3 months in humans. Incubation periods
as short as four days and longer than six years have been documented,
depending on the location and severity of the contaminated wound and the
amount of virus introduced. Initial signs and symptoms of rabies are often nonspecific such as fever and headache. As rabies progresses and causes inflammation of the brain and/or meninges, signs and symptoms can include slight or partial paralysis, anxiety, insomnia, confusion, agitation, abnormal behavior, paranoia, terror, and hallucinations, progressing to delirium, and coma. The person may also have hydrophobia.
Death usually occurs 2 to 10 days after first symptoms. Survival is almost unknown once symptoms have presented, even with the administration of proper and intensive care.
Hydrophobia
A rabid dog
Hydrophobia ("fear of water") is the historic name for rabies.
It refers to a set of symptoms in the later stages of an infection in
which the person has difficulty swallowing, shows panic when presented
with liquids to drink, and cannot quench their thirst. Any mammal
infected with the virus may demonstrate hydrophobia.
Saliva production is greatly increased, and attempts to drink, or
even the intention or suggestion of drinking, may cause excruciatingly
painful spasms of the muscles in the throat and larynx. This can be attributed to the fact that the virus multiplies and assimilates in the salivary glands
of the infected animal with the effect of further transmission through
biting. The ability to transmit the virus would decrease significantly
if the infected individual could swallow saliva and water.
Hydrophobia is commonly associated with furious rabies, which
affects 80% of rabies-infected people. The remaining 20% may experience a
paralytic form of rabies that is marked by muscle weakness, loss of
sensation, and paralysis; this form of rabies does not usually cause
fear of water.
Cause
TEM micrograph with numerous rabies virions (small, dark grey, rodlike particles) and Negri bodies (the larger pathognomonic cellular inclusions of rabies infection)
Rabies is caused by a number of lyssaviruses including the rabies virus and Australian bat lyssavirus.
The rabies virus is the type species of the Lyssavirus genus, in the family Rhabdoviridae, order Mononegavirales. Lyssavirions have helical symmetry, with a length of about 180 nm and a cross-section of about 75 nm. These virions are enveloped and have a single-stranded RNA genome with negative sense. The genetic information is packed as a ribonucleoprotein complex in which RNA is tightly bound by the viral nucleoprotein. The RNA genome
of the virus encodes five genes whose order is highly conserved:
nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein
(G), and the viral RNA polymerase (L).
Once within a muscle or nerve cell, the virus undergoes
replication. The trimeric spikes on the exterior of the membrane of the
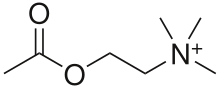
virus interact with a specific cell receptor, the most likely one being
the acetylcholine receptor. The cellular membrane pinches in a procession known as pinocytosis and allows entry of the virus into the cell by way of an endosome.
The virus then uses the acidic environment, which is necessary, of that
endosome and binds to its membrane simultaneously, releasing its five
proteins and single strand RNA into the cytoplasm.
The L protein then transcribes five mRNA strands and a positive
strand of RNA all from the original negative strand RNA using free
nucleotides in the cytoplasm. These five mRNA strands are then
translated into their corresponding proteins (P, L, N, G and M proteins)
at free ribosomes in the cytoplasm. Some proteins require
post-translative modifications. For example, the G protein travels
through the rough endoplasmic reticulum, where it undergoes further folding, and is then transported to the Golgi apparatus, where a sugar group is added to it (glycosylation).
Where there are enough proteins, the viral polymerase will begin
to synthesize new negative strands of RNA from the template of the
positive strand RNA. These negative strands will then form complexes
with the N, P, L and M proteins and then travel to the inner membrane of
the cell, where a G protein has embedded itself in the membrane. The G
protein then coils around the N-P-L-M complex of proteins taking some of
the host cell membrane with it, which will form the new outer envelope
of the virus particle. The virus then buds from the cell.
From the point of entry, the virus is neurotropic, traveling along the neural pathways into the central nervous system.
The virus usually first infects muscle cells close to the site of
infection, where they are able to replicate without being 'noticed' by
the host's immune system. Once enough virus has been replicated, they
begin to bind to acetylcholine receptors (p75NR) at the neuromuscular junction. The virus then travels through the nerve cell axon via retrograde transport, as its P protein interacts with dynein,
a protein present in the cytoplasm of nerve cells. Once the virus
reaches the cell body it travels rapidly to the central nervous system
(CNS), replicating in motor neurons and eventually reaching the brain.
After the brain is infected, the virus travels centrifugally to the
peripheral and autonomic nervous systems, eventually migrating to the
salivary glands, where it is ready to be transmitted to the next host.
Transmission
All warm-blooded species, including humans, may become infected with the rabies virus and develop symptoms. Birds
were first artificially infected with rabies in 1884; however, infected
birds are largely, if not wholly, asymptomatic, and recover. Other bird species have been known to develop rabies antibodies, a sign of infection, after feeding on rabies-infected mammals.
The virus has also adapted to grow in cells of cold-blooded vertebrates. Most animals can be infected by the virus and can transmit the disease to humans. Infected bats, monkeys, raccoons, foxes, skunks, cattle, wolves, coyotes, dogs, cats, and mongooses (normally either the small Asian mongoose or the yellow mongoose) present the greatest risk to humans.
Rabies may also spread through exposure to infected bears, domestic farm animals, groundhogs, weasels, and other wild carnivorans. However, lagomorphs, such as hares and rabbits, and small rodents such as chipmunks, gerbils, guinea pigs, hamsters, mice, rats, and squirrels, are almost never found to be infected with rabies and are not known to transmit rabies to humans.
Bites from mice, rats, or squirrels rarely require rabies prevention
because these rodents are typically killed by any encounter with a
larger, rabid animal, and would, therefore, not be carriers. The Virginia opossum is resistant but not immune to rabies.
The virus is usually present in the nerves and saliva of a symptomatic rabid animal. The route of infection
is usually, but not always, by a bite. In many cases, the infected
animal is exceptionally aggressive, may attack without provocation, and
exhibits otherwise uncharacteristic behavior. This is an example of a viral pathogen modifying the behavior of its host to facilitate its transmission to other hosts.
Transmission between humans is extremely rare. A few cases have been recorded through transplant surgery.
The only well-documented cases of rabies caused by human-to-human
transmission occurred among eight recipients of transplanted corneas and
among three recipients of solid organs.
In addition to transmission from cornea and organ transplants, bite and
non-bite exposures inflicted by infected humans could theoretically
transmit rabies, but no such cases have been documented, since infected
humans are usually hospitalized and necessary precautions taken. Casual
contact, such as touching a person with rabies or contact with
non-infectious fluid or tissue (urine, blood, feces) does not constitute
an exposure and does not require post-exposure prophylaxis.
Additionally, as the virus is present in sperm or vaginal secretions,
spread through sex may be possible.
After a typical human infection by bite, the virus enters the peripheral nervous system. It then travels along the afferent nerves toward the central nervous system.
During this phase, the virus cannot be easily detected within the host,
and vaccination may still confer cell-mediated immunity to prevent
symptomatic rabies. When the virus reaches the brain, it rapidly causes encephalitis,
the prodromal phase, which is the beginning of the symptoms. Once the
patient becomes symptomatic, treatment is almost never effective and
mortality is over 99%. Rabies may also inflame the spinal cord, producing transverse myelitis.
Diagnosis
Rabies can be difficult to diagnose, because, in the early stages, it
is easily confused with other diseases or with aggressiveness. The reference method for diagnosing rabies is the fluorescent antibody test (FAT), an immunohistochemistry procedure, which is recommended by the World Health Organization (WHO).
The FAT relies on the ability of a detector molecule (usually
fluorescein isothiocyanate) coupled with a rabies-specific antibody,
forming a conjugate, to bind to and allow the visualisation of rabies
antigen using fluorescent microscopy techniques. Microscopic analysis
of samples is the only direct method that allows for the identification
of rabies virus-specific antigen in a short time and at a reduced cost,
irrespective of geographical origin and status of the host. It has to
be regarded as the first step in diagnostic procedures for all
laboratories. Autolysed samples can, however, reduce the sensitivity
and specificity of the FAT. The RT PCR assays proved to be a sensitive and specific tool for routine diagnostic purposes, particularly in decomposed samples or archival specimens.
The diagnosis can be reliably made from brain samples taken after
death. The diagnosis can also be made from saliva, urine, and
cerebrospinal fluid samples, but this is not as sensitive or reliable as brain samples. Cerebral inclusion bodies called Negri bodies are 100% diagnostic for rabies infection but are found in only about 80% of cases. If possible, the animal from which the bite was received should also be examined for rabies.
Some light microscopy
techniques may also be used to diagnose rabies at a tenth of the cost
of traditional fluorescence microscopy techniques, allowing
identification of the disease in less-developed countries.
A test for rabies, known as LN34, is easier to run on a dead animal's
brain and might help determine who does and does not need post-exposure
prevention. The test was developed by the CDC in 2018.
Differential diagnosis
The differential diagnosis in a case of suspected human rabies may initially include any cause of encephalitis, in particular infection with viruses such as herpesviruses, enteroviruses, and arboviruses such as West Nile virus. The most important viruses to rule out are herpes simplex virus type one, varicella zoster virus, and (less commonly) enteroviruses, including coxsackieviruses, echoviruses, polioviruses, and human enteroviruses 68 to 71.
New causes of viral encephalitis are also possible, as was
evidenced by the 1999 outbreak in Malaysia of 300 cases of encephalitis
with a mortality rate of 40% caused by Nipah virus, a newly recognized paramyxovirus.
Likewise, well-known viruses may be introduced into new locales, as is
illustrated by the outbreak of encephalitis due to West Nile virus in
the eastern United States.
Epidemiologic factors, such as season, geographic location, and the
patient's age, travel history, and possible exposure to bites, rodents,
and ticks, may help direct the diagnosis.
Prevention
Almost all human cases of rabies were fatal until a vaccine was developed in 1885 by Louis Pasteur and Émile Roux.
Their original vaccine was harvested from infected rabbits, from which
the virus in the nerve tissue was weakened by allowing it to dry for
five to ten days.
Similar nerve tissue-derived vaccines are still used in some countries,
as they are much cheaper than modern cell culture vaccines.
The human diploid cell rabies vaccine was started in 1967. Less expensive purified chicken embryo cell vaccine and purified vero cell rabies vaccine are now available. A recombinant vaccine
called V-RG has been used in Belgium, France, Germany, and the United
States to prevent outbreaks of rabies in undomesticated animals.
Immunization before exposure has been used in both human and nonhuman
populations, where, as in many jurisdictions, domesticated animals are
required to be vaccinated.
The Missouri Department of Health and Senior Services
Communicable Disease Surveillance 2007 Annual Report states the
following can help reduce the risk of contracting rabies:
- Vaccinating dogs, cats, and ferrets against rabies
- Keeping pets under supervision
- Not handling wild animals or strays
- Contacting an animal control officer upon observing a wild animal or a stray, especially if the animal is acting strangely
- If bitten by an animal, washing the wound with soap and water for 10 to 15 minutes and contacting a healthcare provider to determine if post-exposure prophylaxis is required
28 September is World Rabies Day, which promotes the information, prevention, and elimination of the disease.
Vaccinating other animals
In Asia and in parts of the Americas and Africa, dogs remain the
principal host. Mandatory vaccination of animals is less effective in
rural areas. Especially in developing countries, pets may not be
privately kept and their destruction may be unacceptable. Oral vaccines
can be safely distributed in baits, a practice that has successfully
reduced rabies in rural areas of Canada, France, and the United States. In Montreal,
Quebec, Canada, baits are successfully used on raccoons in the
Mount-Royal Park area. Vaccination campaigns may be expensive, and
cost-benefit analysis suggests baits may be a cost-effective method of
control. In Ontario, a dramatic drop in rabies was recorded when an aerial bait-vaccination campaign was launched.
The number of recorded human deaths from rabies in the United
States has dropped from 100 or more annually in the early 20th century
to one or two per year due to widespread vaccination of domestic dogs
and cats and the development of human vaccines and immunoglobulin
treatments. Most deaths now result from bat bites, which may go
unnoticed by the victim and hence untreated.
Treatment
Treatment after exposure can prevent the disease if administered promptly, generally within 10 days of infection.
Thoroughly washing the wound as soon as possible with soap and water
for approximately five minutes is effective in reducing the number of
viral particles. Povidone-iodine or alcohol is then recommended to reduce the virus further.
In the US, the Centers for Disease Control and Prevention recommends people receive one dose of human rabies immunoglobulin (HRIG) and four doses of rabies vaccine over a 14-day period.
The immunoglobulin dose should not exceed 20 units per kilogram body
weight. HRIG is expensive and constitutes most of the cost of post
exposure treatment, ranging as high as several thousand dollars.
As much as possible of this dose should be injected around the bites,
with the remainder being given by deep intramuscular injection at a site
distant from the vaccination site.
The first dose of rabies vaccine is given as soon as possible
after exposure, with additional doses on days 3, 7 and 14 after the
first. Patients who have previously received pre-exposure vaccination do
not receive the immunoglobulin, only the postexposure vaccinations on
days 0 and 3.
The pain and side effects of modern cell-based vaccines
are similar to flu shots. The old nerve-tissue-based vaccinations that
require multiple painful injections into the abdomen with a large needle
are inexpensive, but are being phased out and replaced by affordable
World Health Organization intradermal-vaccination regimens.
Intramuscular vaccination should be given into the deltoid, not the gluteal area,
which has been associated with vaccination failure due to injection
into fat rather than muscle. In infants, the lateral thigh is
recommended.
Awakening to find a bat in the room, or finding a bat in the room
of a previously unattended child or mentally disabled or intoxicated
person, is an indication for post-exposure prophylaxis
(PEP). The recommendation for the precautionary use of PEP in bat
encounters where no contact is recognized has been questioned in the
medical literature, based on a cost–benefit analysis.
However, a 2002 study has supported the protocol of precautionary
administering of PEP where a child or mentally compromised individual
has been alone with a bat, especially in sleep areas, where a bite or
exposure may occur with the victim being unaware. Begun with little or no delay, PEP is 100% effective against rabies.
In the case in which there has been a significant delay in
administering PEP, the treatment should be administered regardless, as
it may still be effective.
Every year, more than 15 million people get vaccination after potential
exposure. While this works well, the cost is significant.
Milwaukee protocol
The Milwaukee protocol, sometimes referred to as the Wisconsin protocol, is a method of attempted treatment of rabies infection in a human being. The treatment involves putting the person into a chemically induced coma and giving antiviral drugs. Jeanna Giese, who in 2004 was the first patient treated with the Milwaukee protocol, became the first person ever recorded to have survived rabies without receiving successful post-exposure prophylaxis. An intention-to-treat analysis has since found this protocol has a survival rate of about 8%. The protocol is not an effective treatment for rabies and its use is not recommended.
Prognosis
In unvaccinated humans, rabies is almost always fatal after neurological symptoms have developed.
Vaccination
after exposure, PEP, is highly successful in preventing the disease if
administered promptly, in general within 6 days of infection. Begun with
little or no delay, PEP is 100% effective against rabies. In the case of significant delay in administering PEP, the treatment still has a chance of success.
Epidemiology
Deaths from rabies per million persons in 2012
0
1
2–4
5–9
10–17
18–69
Rabies-free countries (in green) as of 2010.
always rabies-free rabies eliminated before 1990 rabies eliminated in or after 1990 year of rabies elimination unknown
In 2010, an estimated 26,000 people died from rabies, down from 54,000 in 1990. The majority of the deaths occurred in Asia and Africa. As of 2015, India, followed by China (approximately 6,000), and the Democratic Republic of the Congo (5,600) had the most cases.
A 2015 collaboration between the World Health Organization, World
Organization of Animal Health (OIE), Food and Agriculture Organization
of the United Nation (FAO), and Global Alliance for Rabies Control has a
goal of eliminating deaths from rabies by 2030.
India
India has the highest rate of human rabies in the world, primarily because of stray dogs, whose number has greatly increased since a 2001 law forbade the killing of dogs. Effective control and treatment of rabies in India is hindered by a form of mass hysteria known as puppy pregnancy syndrome
(PPS). Dog bite victims with PPS, male as well as female, become
convinced that puppies are growing inside them, and often seek help from
faith healers rather than medical services. An estimated 20,000 people die every year from rabies in India, more than a third of the global total.
Australia
The rabies virus survives in widespread, varied, rural animal reservoirs. Despite Australia's official rabies-free status, Australian bat lyssavirus
(ABLV), discovered in 1996, is a strain of rabies prevalent in native
bat populations. There have been three human cases of ABLV in Australia,
all of them fatal.
North America
While canine-specific rabies does not circulate among dogs, about a
hundred dogs become infected from other wildlife per year in the US. Rabies is common among wild animals in the United States. Bats, raccoons, skunks and foxes
account for almost all reported cases (98% in 2009). Rabid bats are
found in all 48 contiguous states. Other reservoirs are more limited
geographically; for example, the raccoon rabies virus variant is only
found in a relatively narrow band along the East Coast. Due to a high
public awareness of the virus, efforts at vaccination of domestic
animals and curtailment of feral populations, and availability of postexposure prophylaxis,
incidence of rabies in humans is very rare. A total of 49 cases of the
disease was reported in the country between 1995 and 2011; of these, 11
are thought to have been acquired abroad. Almost all domestically
acquired cases are attributed to bat bites.
Europe
Either no or very few cases of rabies are reported each year in Europe; cases are contracted both during travel and in Europe.
In Switzerland the disease was virtually eliminated after
scientists placed chicken heads laced with live attenuated vaccine in
the Swiss Alps.
The foxes of Switzerland, proven to be the main source of rabies in the
country, ate the chicken heads and immunized themselves.
Italy, after being declared rabies-free from 1997 to 2008, has witnessed a reemergence of the disease in wild animals in the Triveneto regions (Trentino-Alto Adige/Südtirol, Veneto and Friuli-Venezia Giulia), due to the spreading of an epidemic in the Balkans
that also affected Austria. An extensive wild animal vaccination
campaign eliminated the virus from Italy again, and it regained the
rabies-free country status in 2013, the last reported case of rabies
being reported in a red fox in early 2011.
Great Britain has been free of rabies since the beginning of the twentieth century except for a rabies-like virus in a few Daubenton's bats;
there has been one, fatal, case of transmission to a human. There have
been four deaths from rabies, transmitted abroad by dog bite, since
2000.
The last infection in the UK occurred in 1922, and the last death from
indigenous rabies was in 1902. Unlike the other countries of Europe it is protected by being an island, and by strict quarantine procedures.
History
A woodcut from the Middle Ages showing a rabid dog.
François Boissier de Sauvages de Lacroix, Della natura e causa della rabbia (Dissertation sur la nature et la cause de la Rage), 1777
Rabies has been known since around 2000 B.C. The first written record of rabies is in the Mesopotamian Codex of Eshnunna
(circa 1930 BC), which dictates that the owner of a dog showing
symptoms of rabies should take preventive measure against bites. If
another person were bitten by a rabid dog and later died, the owner was
heavily fined.
Ineffective folk remedies abounded in the medical literature of the ancient world. The physician Scribonius Largus prescribed a poultice of cloth and hyena skin; Antaeus recommended a preparation made from the skull of a hanged man.
Rabies appears to have originated in the Old World, the first epizootic in the New World occurring in Boston in 1768.
It spread from there, over the next few years, to various other states,
as well as to the French West Indies, eventually becoming common all
across North America.
Rabies was considered a scourge for its prevalence in the 19th century. In France and Belgium, where Saint Hubert was venerated, the "St Hubert's Key" was heated and applied to cauterize the wound. By an application of magical thinking,
dogs were branded with the key in hopes of protecting them from rabies.
The fear of rabies was almost irrational, due to the number of vectors
(mostly rabid dogs) and the absence of any efficacious treatment. It was
not uncommon for a person bitten by a dog merely suspected of being
rabid to commit suicide or to be killed by others.
In ancient times the attachment of the tongue (the lingual frenulum,
a mucous membrane) was cut and removed as this was where rabies was
thought to originate. This practice ceased with the discovery of the
actual cause of rabies. Louis Pasteur's 1885 nerve tissue vaccine was successful, and was progressively improved to reduce often severe side-effects.
In modern times, the fear of rabies has not diminished, and the
disease and its symptoms, particularly agitation, have served as an inspiration for several works of zombie
or similarly-themed fiction, often portraying rabies as having mutated
into a stronger virus which fills humans with murderous rage or
incurable illness, bringing about a devastating, widespread pandemic.
Milwaukee protocol
The Milwaukee protocol was developed and named by Rodney Willoughby, Jr., following its use in the treatment of Jeanna Giese. Giese, a teenager from Wisconsin, became the first patient known to have survived rabies without receiving the rabies vaccine. It is unclear precisely why Giese survived,
but her case led to sustained and heavy advocacy for the Milwaukee
protocol. Subsequent medical research determined that the Milwaukee
protocol is not an effective treatment for rabies infection, and its use
is not recommended.
Etymology
The term is derived from the Latin rabies, "madness". This, in turn, may be related to the Sanskrit rabhas, "to rage". The Greeks derived the word lyssa, from lud or "violent"; this root is used in the genus name of the rabies virus, Lyssavirus.
Other animals
Rabies is infectious to mammals;
three stages of central nervous system infection are recognized. The
first stage is a one- to three-day period characterized by behavioral
changes and is known as the prodromal stage.
The second is the excitative stage, which lasts three to four days.
This stage is often known as "furious rabies" for the tendency of the
affected animal to be hyper-reactive to external stimuli and bite at
anything near. The third is the paralytic stage and is caused by damage
to motor neurons. Incoordination is seen, owing to rear limb paralysis, and drooling and difficulty swallowing is caused by paralysis of facial and throat muscles. Death is usually caused by respiratory arrest.
Research
The outer shell of the rabies virus, stripped of its RNA contents and thus unable to cause disease, may be used as a vector for the delivery of unrelated genetic material in a research setting. It has the advantage over other pseudotyping methods for gene delivery that the cell targeting (tissue tropism) is more specific for the central nervous system,
a difficult-to-reach site, obviating the need for invasive delivery
methods. It is also capable of infecting neighboring "upstream" cells,
moving from one cell to axons of the next at synapses, and is thus used for retrograde tracing in neuronal circuits.
Evidence indicates artificially increasing the permeability of the blood–brain barrier, which normally does not allow most immune cells across, promotes viral clearance.