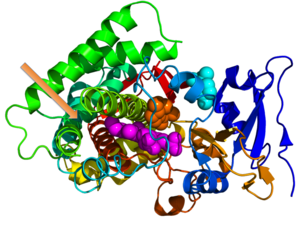
Crystallographic structure of cytochrome P450 from the bacteria S. coelicolor (rainbow colored cartoon, N-terminus = blue, C-terminus = red) complexed with heme cofactor (magenta spheres)
and two molecules of its endogenous substrate epi-isozizaene as orange
and cyan spheres respectively. The orange-colored substrate resides in
the monooxygenase site while the cyan-colored substrate occupies the substrate entrance site. An unoccupied moonlighting terpene synthase site is designated by the orange arrow.
Protein moonlighting (or gene sharing) is a phenomenon by which a protein can perform more than one function. Ancestral moonlighting proteins originally possessed a single function but through evolution, acquired additional functions. Many proteins that moonlight are enzymes; others are receptors, ion channels or chaperones. The most common primary function of moonlighting proteins is enzymatic catalysis,
but these enzymes have acquired secondary non-enzymatic roles. Some
examples of functions of moonlighting proteins secondary to catalysis
include signal transduction, transcriptional regulation, apoptosis, motility, and structural.
Protein moonlighting may occur widely in nature. Protein
moonlighting through gene sharing differs from the use of a single gene
to generate different proteins by alternative RNA splicing, DNA rearrangement, or post-translational processing.
It is also different from multifunctionality of the protein, in which
the protein has multiple domains, each serving a different function.
Protein moonlighting by gene sharing means that a gene may acquire and
maintain a second function without gene duplication and without loss of
the primary function. Such genes are under two or more entirely
different selective constraints.
Various techniques have been used to reveal moonlighting
functions in proteins. The detection of a protein in unexpected
locations within cells, cell types, or tissues may suggest that a
protein has a moonlighting function. Furthermore, sequence or structure
homology of a protein may be used to infer both primary function as
well as secondary moonlighting functions of a protein.
The most well-studied examples of gene sharing are crystallins.
These proteins, when expressed at low levels in many tissues function
as enzymes, but when expressed at high levels in eye tissue, become
densely packed and thus form lenses. While the recognition of gene
sharing is relatively recent—the term was coined in 1988, after
crystallins in chickens and ducks were found to be identical to
separately identified enzymes—recent studies have found many examples
throughout the living world. Joram Piatigorsky has suggested that many or all proteins exhibit gene sharing to some extent, and that gene sharing is a key aspect of molecular evolution. The genes encoding crystallins must maintain sequences for catalytic function and transparency maintenance function.
Inappropriate moonlighting is a contributing factor in some
genetic diseases, and moonlighting provides a possible mechanism by
which bacteria may become resistant to antibiotics.
Discovery
The
first observation of a moonlighting protein was made in the late 1980s
by Joram Piatigorsky and Graeme Wistow during their research on crystallin
enzymes. Piatigorsky determined that lens crystallin conservation and
variance is due to other moonlighting functions outside of the lens. Originally Piatigorsky called these proteins "gene sharing" proteins, but the colloquial description moonlighting was subsequently applied to proteins by Constance Jeffery in 1999 to draw a similarity between multitasking proteins and people who work two jobs. The phrase "gene sharing" is ambiguous since it is also used to describe horizontal gene transfer, hence the phrase "protein moonlighting" has become the preferred description for proteins with more than one function.
Evolution
It is believed that moonlighting proteins came about by means of evolution
through which uni-functional proteins gained the ability to perform
multiple functions. With alterations, much of the protein's unused space
can provide new functions. Many moonlighting proteins are the result of gene fusion of two single function genes.
Alternatively a single gene can acquire a second function since the
active site of the encoded protein typically is small compared to the
overall size of the protein leaving considerable room to accommodate a
second functional site. In yet a third alternative, the same active site
can acquire a second function through mutations of the active site.
The development of moonlighting proteins may be evolutionarily
favorable to the organism since a single protein can do the job of
multiple proteins conserving amino acids and energy required to
synthesize these proteins. However, there is no universally agreed upon theory that explains why proteins with multiple roles evolved.
While using one protein to perform multiple roles seems advantageous
because it keeps the genome small, we can conclude that this is probably
not the reason for moonlighting because of the large of amount of noncoding DNA.
Functions
Many proteins catalyze a chemical reaction.
Other proteins fulfill structural, transport, or signaling roles.
Furthermore, numerous proteins have the ability to aggregate into supramolecular assemblies. For example, a ribosome is made up of 90 proteins and RNA.
A number of the currently known moonlighting proteins are evolutionarily derived from highly conserved
enzymes, also called ancient enzymes. These enzymes are frequently
speculated to have evolved moonlighting functions. Since highly
conserved proteins are present in many different organisms, this
increases the chance that they would develop secondary moonlighting
functions. A high fraction of enzymes involved in glycolysis,
an ancient universal metabolic pathway, exhibit moonlighting behavior.
Furthermore, it has been suggested that as many as 7 out of 10 proteins
in glycolysis and 7 out of 8 enzymes of the tricarboxylic acid cycle
exhibit moonlighting behavior.
An example of a moonlighting enzyme is pyruvate carboxylase. This enzyme catalyzes the carboxylation of pyruvate into oxaloacetate, thereby replenishing the tricarboxylic acid cycle. Surprisingly, in yeast species such as H. polymorpha and P. pastoris, pyruvate carboylase is also essential for proper targeting and assembly of the peroxisomal protein alcohol oxidase (AO). AO, the first enzyme of methanol metabolism, is a homo-octameric flavoenzyme. In wild type cells, this enzyme is present as enzymatically active AO octamers in the peroxisomal
matrix. However, in cells lacking pyruvate carboxylase, AO monomers
accumulate in the cytosol, indicating that pyruvate carboxylase has a
second fully unrelated function in assembly and import. The function in
AO import/assembly is fully independent of the enzyme activity of
pyruvate carboxylase, because amino acid substitutions can be introduced
that fully inactive the enzyme activity of pyruvate carboxylase,
without affecting its function in AO assembly and import. Conversely,
mutations are known that block the function of this enzyme in import and
assembly of AO, but have no effect on the enzymatic activity of the
protein.
The E. coli anti-oxidant thioredoxin protein is another example of a moonlighting protein. Upon infection with the bacteriophage T7, E. coli thioredoxin forms a complex with T7 DNA polymerase,
which results in enhanced T7 DNA replication, a crucial step for
successful T7 infection. Thioredoxin binds to a loop in T7 DNA
polymerase to bind more strongly to the DNA. The anti-oxidant function
of thioredoxin is fully autonomous and fully independent of T7 DNA
replication, in which the protein most likely fulfills the functional
role.
ADT2 and ADT5 are another example of moonlighting proteins found
in plants. Both of these proteins have roles in phenylalanine
biosynthesis like all other ADTs. However ADT2, together with FtsZ is
necessary in chloroplast division and ADT5 is transported by stromules
into the nucleus.
| Kingdom | Protein | Organism | Function | |
|---|---|---|---|---|
| primary | moonlighting | |||
| Animal | ||||
| Aconitase | H. sapiens | TCA cycle enzyme | Iron homeostasis | |
| ATF2 | H. sapiens | Transcription factor | DNA damage response | |
| Crystallins | Various | Lens structural protein | Various enzyme | |
| Cytochrome c | Various | Energy metabolism | Apoptosis | |
| DLD | H. sapiens | Energy metabolism | Protease | |
| ERK2 | H. sapiens | MAP kinase | Transcriptional repressor | |
| ESCRT-II complex | D. melanogaster | Endosomal protein sorting | Bicoid mRNA localization | |
| STAT3 | M. musculus | Transcription factor | Electron transport chain | |
| Plant | ||||
| Hexokinase | A. thaliana | Glucose metabolism | Glucose signaling / cell death control | |
| Presenilin | P. patens | γ-secretase | Cystoskeletal function | |
| Fungus | ||||
| Aconitase | S. cerevisiae | TCA cycle enzyme | mtDNA stability | |
| Aldolase | S. cerevisiae | Glycolytic enzyme | V-ATPase assembly | |
| Arg5,6 | S. cerevisiae | Arginine biosynthesis | Transcriptional control | |
| Enolase | S. cerevisiae | Glycolytic enzyme |
Homotypic vacuole fusion Mitochondrial tRNA import | |
| Galactokinase | K. lactis | Galactose catabolism enzyme | Induction galactose genes | |
| Hal3 | S. cerevisiae | Halotolerance determinant | Coenzyme A biosynthesis | |
| HSP60 | S. cerevisiae | Mitochondrial chaperone | Stabilization active DNA ori's | |
| Phosphofructokinase | P. pastoris | Glycolytic enzyme | Autophagy peroxisomes | |
| Pyruvate carboxylase | H. polymorpha | Anaplerotic enzyme | Assembly of alcohol oxidase | |
| Vhs3 | S. cerevisiae | Halotolerance determinant | Coenzyme A biosynthesis | |
| Prokaryotes | ||||
| Aconitase | M. tuberculosis | TCA cycle enzyme | Iron-responsive protein | |
| CYP170A1 | S. coelicolor | Albaflavenone synthase | Terpene synthase | |
| Enolase | S. pneumoniae | Glycolytic enzyme | Plasminogen binding | |
| GroEL | E. aerogenes | Chaperone | Insect toxin | |
| Glutamate racemase (MurI) | E. coli | cell wall biosynthesis | gyrase inhibition | |
| Thioredoxin | E. coli | Anti-oxidant | T7 DNA polymerase subunit | |
| Protist | ||||
| Aldolase | P. vivax | Glycolytic enzyme | Host-cell invasion | |
Mechanisms
Crystallographic structure of aconitase
In many cases, the functionality of a protein not only depends on its
structure, but also its location. For example, a single protein may
have one function when found in the cytoplasm of a cell, a different
function when interacting with a membrane, and yet a third function if
excreted from the cell. This property of moonlighting proteins is known
as "differential localization". For example, in higher temperatures DegP (HtrA) will function as a protease by the directed degradation of proteins and in lower temperatures as a chaperone by assisting the non-covalent folding or unfolding and the assembly or disassembly of other macromolecular structures.
Furthermore, moonlighting proteins may exhibit different behaviors not
only as a result of its location within a cell, but also the type of
cell that the protein is expressed in. Multifunctionality could also be as a consequence of differential post translational modifications (PTM'S). In the case of the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH)alterations in the PTM's have been shown to be associated with higher order multi functionality.
Other methods through which proteins may moonlight are by changing their oligomeric state, altering concentrations of the protein's ligand or substrate, use of alternative binding sites, or finally through phosphorylation. An example of a protein that displays different function in different oligomeric states is pyruvate kinase which exhibits metabolic activity as a tetramer and thyroid hormone–binding
activity as a monomer. Changes in the concentrations of ligands or
substrates may cause a switch in protein a protein's function. For
example, in the presence of low iron concentrations, aconitase functions as an enzyme while at high iron concentration, aconitase functions as an iron-responsive element-binding protein
(IREBP). Proteins may also perform separate functions through the use
of alternative binding sites that perform different tasks. An example of
this is ceruloplasmin, a protein that functions as an oxidase in copper metabolism and moonlights as a copper-independent glutathione peroxidase.
Lastly, phosphorylation may sometimes cause a switch in the function of
a moonlighting protein. For example, phosphorylation of phosphoglucose isomerase (PGI) at Ser-185 by protein kinase CK2 causes it to stop functioning as an enzyme, while retaining its function as an autocrine motility factor.
Hence when a mutation takes place that inactivates a function of a
moonlighting proteins, the other function(s) are not necessarily
affected.
The crystal structures of several moonlighting proteins, such as I-AniI homing endonuclease / maturase and the PutA proline dehydrogenase / transcription factor, have been determined.
An analysis of these crystal structures has demonstrated that
moonlighting proteins can either perform both functions at the same
time, or through conformational changes,
alternate between two states, each of which is able to perform a
separate function. For example, the protein DegP plays a role in
proteolysis with higher temperatures and is involved in refolding
functions at lower temperatures.
Lastly, these crystal structures have shown that the second function
may negatively affect the first function in some moonlighting proteins.
As seen in ƞ-crystallin, the second function of a protein can alter the
structure, decreasing the flexibility, which in turn can impair
enzymatic activity somewhat.
Identification methods
Moonlighting
proteins have usually been identified by chance because there is no
clear procedure to identify secondary moonlighting functions. Despite
such difficulties, the number of moonlighting proteins that have been
discovered is rapidly increasing. Furthermore, moonlighting proteins
appear to be abundant in all kingdoms of life.
Various methods have been employed to determine a protein's
function including secondary moonlighting functions. For example, the
tissue, cellular, or subcellular distribution of a protein may provide
hints as to the function. Real-time PCR is used to quantify mRNA
and hence infer the presence or absence of a particular protein which
is encoded by the mRNA within different cell types. Alternatively immunohistochemistry or mass spectrometry
can be used to directly detect the presence of proteins and determine
in which subcellular locations, cell types, and tissues a particular
protein is expressed.
Mass spectrometry may be used to detect proteins based on their mass-to-charge ratio. Because of alternative splicing and posttranslational modification, identification of proteins based on the mass of the parent ion alone is very difficult. However tandem mass spectrometry
in which each of the parent peaks is in turn fragmented can be used to
unambiguously identify proteins. Hence tandem mass spectrometry is one
of the tools used in proteomics
to identify the presence of proteins in different cell types or
subcellular locations. While the presence of a moonlighting protein in
an unexpected location may complicate routine analyses, at the same
time, the detection of a protein in unexpected multiprotein complexes or
locations suggests that protein may have a moonlighting function.
Furthermore, mass spectrometry may be used to determine if a protein
has high expression levels that do not correlate to the enzyme's
measured metabolic activity. These expression levels may signify that
the protein is performing a different function than previously known.
The structure
of a protein can also help determine its functions. Protein structure
in turn may be elucidated with various techniques including X-ray crystallography or NMR. Dual polarization interferometry
may be used to measure changes in protein structure which may also give
hints to the protein's function. Finally, application of systems biology approaches such as interactomics give clues to a proteins function based on what it interacts with.
Higher order multifunctionality
In the case of the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase
(GAPDH), in addition to the large number of alternate functions it has
also been observed that it can be involved in the same function by
multiple means (multifunctionality within multifunctionality). For
example, in its role in maintenance of cellular iron homeostasis GAPDH
can function to import or extrude iron from cells. Moreover, in case of
its iron import activities it can traffic into cells holo-transferrin
as well as the related molecule lactoferrin by multiple pathways.
Example
Crystallins
A crystallin from ducks that exhibits argininosuccinate lyase activity and is a key structural component in eye lenses, an example of gene sharing
In the case of crystallins, the genes must maintain sequences for catalytic function and transparency maintenance function.
The abundant lens crystallins have been generally viewed as static
proteins serving a strictly structural role in transparency and cataract.
However, recent studies have shown that the lens crystallins are much
more diverse than previously recognized and that many are related or
identical to metabolic enzymes and stress proteins found in numerous
tissues. Unlike other proteins performing highly specialized tasks, such as globin or rhodopsin,
the crystallins are very diverse and show numerous species differences.
Essentially all vertebrate lenses contain representatives of the α and
β/γ crystallins, the "Ubiquitous crystallins", which are themselves
heterogeneous, and only few species or selected taxonomic groups use
entirely different proteins as lens crystallins.This paradox of
crystallins being highly conserved in sequence while extremely diverse
in number and distribution shows that many crystallins have vital
functions outside the lens and cornea, and this multi-functionality of
the crystallins is achieved by gene sharing.
Gene regulation
Crystallin recruitment may occur by changes in gene regulation
that leads to high lens expression. One such example is gluthathione
S-transferase/S11-crystallin that was specialized for lens expression by
change in gene regulation and gene duplication.
The fact that similar transcriptional factors such as Pax-6, and
retinoic acid receptors, regulate different crystalline genes, suggests
that lens-specific expression have played a crucial role for recruiting
multifunctional protein as crystallins. Crystallin recruitment has
occurred both with and without gene duplication, and tandem gene
duplication has taken place among some of the crystallins with one of
the duplicates specializing for lens expression. Ubiquitous α
–crystallins and bird δ –crystallins are two examples.
Alpha crystallins
The α-crystallins, which contributed to the discovery of crystallins as borrowed proteins,
have continually supported the theory of gene sharing, and helped
delineating the mechanisms used for gene sharing as well. There are two
α-crystallin genes (αA and αB), which are about 55% identical in amino
acid sequence.
Expression studies in non-lens cells showed that the αB-crystallin,
other than being a functional lens protein, is a functional small heat
shock protein. αB-crystallin is induced by heat and other physiological stresses, and it can protect the cells from elevated temperatures and hypertonic stress. αB-crystallin is also over-expressed in many pathologies, including neurodegenerative diseases, fibroblasts of patients with Werner's disease
showing premature senescence, and growth abnormalities. In addition to
being over-expressed under abnormal conditions, αB-crystallin is
constitutively expressed in heart, skeletal muscle, kidney, lung and
many other tissues. In contrast to αB-crystallin, except for low-level expression in the thymus, spleen and retina, αA-crystallin is highly specialized for expression in the lens and is not stress-inducible. However, like αB-crystallin, it can also function as molecular chaperone and protect against thermal stress.
Beta/gamma-crystallins
β/γ-crystallins
are different from α-crystallins in that they are a large multigene
family. Other proteins like bacterial spore coat, a slime mold cyst
protein, and epidermis differentiation-specific protein, contain the
same Greek key motifs and are placed under β/γ crystallin superfamily.
This relationship supports the idea that β/γ- crystallins have been
recruited by a gene-sharing mechanism. However, except for few reports,
non-refractive function of the β/γ-crystallin is yet to be found.
Corneal crystallins
Similar to lens, cornea is a transparent, avascular tissue derived from the ectoderm that is responsible for focusing light onto the retina.
However, unlike lens, cornea depends on the air-cell interface and its
curvature for refraction. Early immunology studies have shown that BCP
54 comprises 20–40% of the total soluble protein in bovine cornea.
Subsequent studies have indicated that BCP 54 is ALDH3, a tumor and
xenobiotic-inducible cytosolic enzyme, found in human, rat, and other
mammals.
Non refractive roles of crystallins in lens and cornea
While
it is evident that gene sharing resulted in many of lens crystallins
being multifunctional proteins, it is still uncertain to what extent the
crystallins use their non-refractive properties in the lens, or on what
basis they were selected. The α-crystallins provide a convincing case
for a lens crystallin using its non-refractive ability within the lens
to prevent protein aggregation under a variety of environmental stresses and to protect against enzyme inactivation by post-translational modifications such as glycation. The α-crystallins may also play a functional role in the stability and remodeling of the cytoskeleton during fiber cell differentiation in the lens. In cornea, ALDH3 is also suggested to be responsible for absorbing UV-B light.
Co-evolution of lens and cornea through gene sharing
Based
on the similarities between lens and cornea, such as abundant
water-soluble enzymes, and being derived from ectoderm, the lens and
cornea are thought to be co-evolved as a "refraction unit." Gene sharing
would maximize light transmission and refraction to the retina by this
refraction unit. Studies have shown that many water-soluble
enzymes/proteins expressed by cornea are identical to taxon-specific
lens crystallins, such as ALDH1A1/ η-crystallin, α-enolase/τ-crystallin,
and lactic dehydrogenase/ -crystallin. Also, the anuran
corneal epithelium, which can transdifferentiate to regenerate the
lens, abundantly expresses ubiquitous lens crystallins, α, β and γ, in
addition to the taxon-specific crystallin α-enolase/τ-crystallin.
Overall, the similarity in expression of these proteins in the cornea
and lens, both in abundance and taxon-specificity, supports the idea of
co-evolution of lens and cornea through gene sharing.
Relationship to similar concepts
Gene
sharing is related to, but distinct from, several concepts in genetics,
evolution, and molecular biology. Gene sharing entails multiple
effects from the same gene, but unlike pleiotropy,
it necessarily involves separate functions at the molecular level. A
gene could exhibit pleiotropy when single enzyme function affects
multiple phenotypic traits; mutations of a shared gene could potentially affect only a single trait. Gene duplication
followed by differential mutation is another phenomenon thought to be a
key element in the evolution of protein function, but in gene sharing,
there is no divergence of gene sequence when proteins take on new
functions; the single polypeptide takes on new roles while retaining old
ones. Alternative splicing
can result in the production of multiple polypeptides (with multiple
functions) from a single gene, but by definition, gene sharing involves
multiple functions of a single polypeptide.
Clinical significance
The multiple roles of moonlighting proteins complicates the determination of phenotype from genotype, hampering the study of inherited metabolic disorders.
The complex phenotypes of several disorders are suspected to be caused by the involvement of moonlighting proteins. The protein GAPDH
has at least 11 documented functions, one of which includes apoptosis.
Excessive apoptosis is involved in many neurodegenerative diseases, such
as Huntington's, Alzheimer's, and Parkinson's as well as in brain ischemia. In one case, GAPDH was found in the degenerated neurons of individuals who had Alzheimer's disease.
Although there is insufficient evidence for definite conclusions,
there are well documented examples of moonlighting proteins that play a
role in disease. One such disease is tuberculosis. One moonlighting protein in the bacterium M. tuberculosis has a function which counteracts the effects of antibiotics. Specifically, M. tuberculosis gains antibiotic resistance against ciprofloxacin from overexpression of Glutamate racemase in vivo.
GAPDH localized to the surface of pathogenic mycobacteriea has been
shown to capture and traffic the mammalian iron carrier protein
transferrin into cells resulting in iron acquisition by the pathogen.