Greenhouse effect schematic showing energy flows between space, the atmosphere, and Earth's surface. Energy influx and emittance are expressed in watts per square meter (W/m2).
A greenhouse gas is a gas that absorbs and emits radiant energy within the thermal infrared range. Greenhouse gases cause the greenhouse effect. The primary greenhouse gases in Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide and ozone. Without greenhouse gases, the average temperature of Earth's surface would be about −18 °C (0 °F), rather than the present average of 15 °C (59 °F). The atmospheres of Venus, Mars and Titan also contain greenhouse gases.
Human activities since the beginning of the Industrial Revolution (around 1750) have produced a 40% increase in the atmospheric concentration of carbon dioxide (CO2), from 280 ppm in 1750 to 406 ppm in early 2017. This increase has occurred despite the uptake of more than half of the emissions by various natural "sinks" involved in the carbon cycle. The vast majority of anthropogenic carbon dioxide emissions (i.e., emissions produced by human activities) come from combustion of fossil fuels, principally coal, oil, and natural gas,
with additional contributions coming from deforestation, changes in
land use, soil erosion and agriculture (including livestock).
Should greenhouse gas emissions continue at their rate in 2017,
Earth's surface temperature could exceed historical values as early as
2047, with potentially harmful effects on ecosystems, biodiversity and human livelihoods. At current emission rates temperatures could increase by 2 °C, which the United Nations' IPCC designated as the upper limit to avoid "dangerous" levels, by 2036.
Gases in Earth's atmosphere
Atmospheric absorption and scattering at different wavelengths of electromagnetic waves. The largest absorption band of carbon dioxide is not far from the maximum in the thermal emission from ground, and it partly closes the window of transparency of water; hence its major effect.
Greenhouse gases
Greenhouse gases are those that absorb and emit infrared radiation in the wavelength range emitted by Earth. In order, the most abundant greenhouse gases in Earth's atmosphere are:
- Water vapor (H
2O) - Carbon dioxide (CO
2) - Methane (CH
4) - Nitrous oxide (N
2O) - Ozone (O
3) - Chlorofluorocarbons (CFCs)
- Hydrofluorocarbons (incl. HCFCs and HFCs)
Atmospheric concentrations are determined by the balance between
sources (emissions of the gas from human activities and natural systems)
and sinks (the removal of the gas from the atmosphere by conversion to a
different chemical compound or absorption by bodies of water). The proportion of an emission remaining in the atmosphere after a specified time is the "airborne fraction" (AF). The annual airborne fraction
is the ratio of the atmospheric increase in a given year to that year's
total emissions. As of 2006 the annual airborne fraction for CO2 was about 0.45. The annual airborne fraction increased at a rate of 0.25 ± 0.21% per year over the period 1959–2006.
Non-greenhouse gases
The major atmospheric constituents, nitrogen (N
2), oxygen (O
2), and argon (Ar), are not greenhouse gases because molecules containing two atoms of the same element such as N
2 and O
2 have no net change in the distribution of their electrical charges when they vibrate, and monatomic gases such as Ar do not have vibrational modes. Hence they are almost totally unaffected by infrared radiation. Some heterodiatomic molecules containing atoms of different elements such as carbon monoxide (CO) or hydrogen chloride (HCl) do absorb infrared radiation, although these molecules are short-lived in the atmosphere owing to their reactivity and solubility. Therefore, they do not contribute significantly to the greenhouse effect and often are omitted when discussing greenhouse gases.
2), oxygen (O
2), and argon (Ar), are not greenhouse gases because molecules containing two atoms of the same element such as N
2 and O
2 have no net change in the distribution of their electrical charges when they vibrate, and monatomic gases such as Ar do not have vibrational modes. Hence they are almost totally unaffected by infrared radiation. Some heterodiatomic molecules containing atoms of different elements such as carbon monoxide (CO) or hydrogen chloride (HCl) do absorb infrared radiation, although these molecules are short-lived in the atmosphere owing to their reactivity and solubility. Therefore, they do not contribute significantly to the greenhouse effect and often are omitted when discussing greenhouse gases.
Indirect radiative effects
The
false colors in this image represent concentrations of carbon monoxide
in the lower atmosphere, ranging from about 390 parts per billion (dark
brown pixels), to 220 parts per billion (red pixels), to 50 parts per
billion (blue pixels).
Some gases have indirect radiative effects (whether or not they are
greenhouse gases themselves). This happens in two main ways. One way is
that when they break down in the atmosphere they produce another
greenhouse gas. For example, methane and carbon monoxide (CO) are
oxidized to give carbon dioxide (and methane oxidation also produces
water vapor). Oxidation of CO to CO2 directly produces an
unambiguous increase in radiative forcing although the reason is subtle.
The peak of the thermal IR emission from Earth's surface is very close
to a strong vibrational absorption band of CO2 (15 microns, or 667 cm−1). On the other hand, the single CO vibrational band only absorbs IR at much shorter wavelengths (4.7 microns, or 2145 cm−1), where the emission of radiant energy from Earth's surface is at least a factor of ten lower. Oxidation of methane to CO2,
which requires reactions with the OH radical, produces an instantaneous
reduction in radiative absorption and emission since CO2 is a weaker greenhouse gas than methane. However, the oxidation of CO and CH
4 are entwined since both consume OH radicals. In any case, the calculation of the total radiative effect includes both direct and indirect forcing.
4 are entwined since both consume OH radicals. In any case, the calculation of the total radiative effect includes both direct and indirect forcing.
A second type of indirect effect happens when chemical reactions
in the atmosphere involving these gases change the concentrations of
greenhouse gases. For example, the destruction of non-methane volatile organic compounds
(NMVOCs) in the atmosphere can produce ozone. The size of the indirect
effect can depend strongly on where and when the gas is emitted.
Methane has indirect effects in addition to forming CO2. The main chemical that reacts with methane in the atmosphere is the hydroxyl radical
(OH), thus more methane means that the concentration of OH goes down.
Effectively, methane increases its own atmospheric lifetime and
therefore its overall radiative effect. The oxidation of methane can
produce both ozone and water; and is a major source of water vapor in
the normally dry stratosphere. CO and NMVOCs produce CO2
when they are oxidized. They remove OH from the atmosphere, and this
leads to higher concentrations of methane. The surprising effect of this
is that the global warming potential of CO is three times that of CO2. The same process that converts NMVOCs to carbon dioxide can also lead to the formation of tropospheric ozone. Halocarbons have an indirect effect because they destroy stratospheric ozone. Finally, hydrogen can lead to ozone production and CH
4 increases as well as producing stratospheric water vapor.
4 increases as well as producing stratospheric water vapor.
Contribution of clouds to Earth's greenhouse effect
The major non-gas contributor to Earth's greenhouse effect, clouds,
also absorb and emit infrared radiation and thus have an effect on
greenhouse gas radiative properties. Clouds are water droplets or ice crystals suspended in the atmosphere.
Impacts on the overall greenhouse effect
Schmidt et al. (2010)
analyzed how individual components of the atmosphere contribute to the
total greenhouse effect. They estimated that water vapor accounts for
about 50% of Earth's greenhouse effect, with clouds contributing 25%,
carbon dioxide 20%, and the minor greenhouse gases and aerosols accounting for the remaining 5%. In the study, the reference model atmosphere is for 1980 conditions. Image credit: NASA.
The contribution of each gas to the greenhouse effect is determined
by the characteristics of that gas, its abundance, and any indirect
effects it may cause. For example, the direct radiative effect of a mass
of methane is about 84 times stronger than the same mass of carbon
dioxide over a 20-year time frame
but it is present in much smaller concentrations so that its total
direct radiative effect is smaller, in part due to its shorter
atmospheric lifetime. On the other hand, in addition to its direct
radiative impact, methane has a large, indirect radiative effect because
it contributes to ozone formation. Shindell et al. (2005) argue that the contribution to climate change from methane is at least double previous estimates as a result of this effect.
When ranked by their direct contribution to the greenhouse effect, the most important are:
| Compound |
Formula |
Concentration in atmosphere (ppm) |
Contribution (%) |
|---|---|---|---|
| Water vapor and clouds | H 2O |
10–50,000(A) | 36–72% |
| Carbon dioxide | CO2 | ~400 | 9–26% |
| Methane | CH 4 |
~1.8 | 4–9% |
| Ozone | O 3 |
2–8(B) | 3–7% |
| notes: (A) Water vapor strongly varies locally (B) The concentration in stratosphere. About 90% of the ozone in Earth's atmosphere is contained in the stratosphere. | |||
In addition to the main greenhouse gases listed above, other greenhouse gases include sulfur hexafluoride, hydrofluorocarbons and perfluorocarbons. Some greenhouse gases are not often listed. For example, nitrogen trifluoride has a high global warming potential (GWP) but is only present in very small quantities.
Proportion of direct effects at a given moment
It
is not possible to state that a certain gas causes an exact percentage
of the greenhouse effect. This is because some of the gases absorb and
emit radiation at the same frequencies as others, so that the total
greenhouse effect is not simply the sum of the influence of each gas.
The higher ends of the ranges quoted are for each gas alone; the lower
ends account for overlaps with the other gases. In addition, some gases, such as methane, are known to have large indirect effects that are still being quantified.
Atmospheric lifetime
Aside from water vapor, which has a residence time of about nine days, major greenhouse gases are well mixed and take many years to leave the atmosphere.
Although it is not easy to know with precision how long it takes
greenhouse gases to leave the atmosphere, there are estimates for the
principal greenhouse gases.
Jacob (1999) defines the lifetime of an atmospheric species X in a one-box model as the average time that a molecule of X remains in the box. Mathematically can
be defined as the ratio of the mass (in kg) of X in the box to its removal rate, which is the sum of the flow of X out of the box
(),
chemical loss of X
(),
and deposition of X
()
(all in kg/s):
.
If output of this gas into the box ceased, then after time , its concentration would decrease by about 63%.
The atmospheric lifetime of a species therefore measures the time
required to restore equilibrium following a sudden increase or decrease
in its concentration in the atmosphere. Individual atoms or molecules
may be lost or deposited to sinks such as the soil, the oceans and other
waters, or vegetation and other biological systems, reducing the excess
to background concentrations. The average time taken to achieve this is
the mean lifetime.
Carbon dioxide has a variable atmospheric lifetime, and cannot be specified precisely. The atmospheric lifetime of CO2 is estimated of the order of 30–95 years.
This figure accounts for CO2 molecules being removed from the
atmosphere by mixing into the ocean, photosynthesis, and other
processes. However, this excludes the balancing fluxes of CO2 into the atmosphere from the geological reservoirs, which have slower characteristic rates. Although more than half of the CO2 emitted is removed from the atmosphere within a century, some fraction (about 20%) of emitted CO2 remains in the atmosphere for many thousands of years. Similar issues apply to other greenhouse gases, many of which have longer mean lifetimes than CO2, e.g. N2O has a mean atmospheric lifetime of 121 years.
Radiative forcing
Earth
absorbs some of the radiant energy received from the sun, reflects some
of it as light and reflects or radiates the rest back to space as heat. Earth's surface temperature depends on this balance between incoming and outgoing energy. If this energy balance is shifted, Earth's surface becomes warmer or cooler, leading to a variety of changes in global climate.
A number of natural and man-made mechanisms can affect the global energy balance and force changes in Earth's climate. Greenhouse gases are one such mechanism.
Greenhouse gases absorb and emit some of the outgoing energy radiated
from Earth's surface, causing that heat to be retained in the lower
atmosphere. As explained above,
some greenhouse gases remain in the atmosphere for decades or even
centuries, and therefore can affect Earth's energy balance over a long
period. Radiative forcing
quantifies the effect of factors that influence Earth's energy balance,
including changes in the concentrations of greenhouse gases.
Positive radiative forcing leads to warming by increasing the net
incoming energy, whereas negative radiative forcing leads to cooling.
Global warming potential
The global warming potential
(GWP) depends on both the efficiency of the molecule as a greenhouse
gas and its atmospheric lifetime. GWP is measured relative to the same mass of CO2 and evaluated for a specific timescale. Thus, if a gas has a high (positive) radiative forcing
but also a short lifetime, it will have a large GWP on a 20-year scale
but a small one on a 100-year scale. Conversely, if a molecule has a
longer atmospheric lifetime than CO2 its GWP will increase when the timescale is considered. Carbon dioxide is defined to have a GWP of 1 over all time periods.
Methane has an atmospheric lifetime of 12 ± 3 years. The 2007 IPCC report lists the GWP as 72 over a time scale of 20 years, 25 over 100 years and 7.6 over 500 years. A 2014 analysis, however, states that although methane's initial impact is about 100 times greater than that of CO2,
because of the shorter atmospheric lifetime, after six or seven
decades, the impact of the two gases is about equal, and from then on
methane's relative role continues to decline. The decrease in GWP at longer times is because methane is degraded to water and CO2 through chemical reactions in the atmosphere.
Examples of the atmospheric lifetime and GWP relative to CO2 for several greenhouse gases are given in the following table:
| Gas name | Chemical formula |
Lifetime (years) |
Global warming potential for given time | ||
|---|---|---|---|---|---|
| 20-yr | 100-yr | 500-yr | |||
| Carbon dioxide | CO2 | 30–95 | 1 | 1 | 1 |
| Methane | CH 4 |
12 | 84 | 28 | 7.6 |
| Nitrous oxide | N 2O |
121 | 264 | 265 | 153 |
| CFC-12 | CCl 2F 2 |
100 | 10 800 | 10 200 | 5 200 |
| HCFC-22 | CHClF 2 |
12 | 5 280 | 1 760 | 549 |
| Tetrafluoromethane | CF 4 |
50 000 | 4 880 | 6 630 | 11 200 |
| Hexafluoroethane | C 2F 6 |
10 000 | 8 210 | 11 100 | 18 200 |
| Sulfur hexafluoride | SF 6 |
3 200 | 17 500 | 23 500 | 32 600 |
| Nitrogen trifluoride | NF 3 |
500 | 12 800 | 16 100 | 20 700 |
The use of CFC-12 (except some essential uses) has been phased out due to its ozone depleting properties. The phasing-out of less active HCFC-compounds will be completed in 2030.
Carbon dioxide in Earth's atmosphere if half of global-warming emissions are not absorbed.
(NASA simulation; 9 November 2015)
(NASA simulation; 9 November 2015)
Nitrogen dioxide 2014 – global air quality levels
(released 14 December 2015).
(released 14 December 2015).
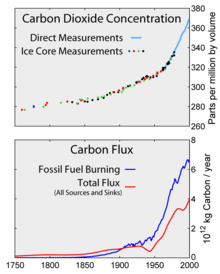
Top: Increasing atmospheric carbon dioxide levels as measured in the atmosphere and reflected in ice cores. Bottom: The amount of net carbon increase in the atmosphere, compared to carbon emissions from burning fossil fuel.
This diagram shows a simplified representation of the contemporary global carbon cycle. Changes are measured in gigatons of carbon per year (GtC/y). Canadell et al. (2007) estimated the growth rate of global average atmospheric CO2 for 2000–2006 as 1.93 parts-per-million per year (4.1 petagrams of carbon per year).
Natural and anthropogenic sources
Aside from purely human-produced synthetic halocarbons, most
greenhouse gases have both natural and human-caused sources. During the
pre-industrial Holocene,
concentrations of existing gases were roughly constant, because the
large natural sources and sinks roughly balanced. In the industrial era,
human activities have added greenhouse gases to the atmosphere, mainly
through the burning of fossil fuels and clearing of forests.
The 2007 Fourth Assessment Report
compiled by the IPCC (AR4) noted that "changes in atmospheric
concentrations of greenhouse gases and aerosols, land cover and solar
radiation alter the energy balance of the climate system", and concluded
that "increases in anthropogenic greenhouse gas concentrations is very
likely to have caused most of the increases in global average
temperatures since the mid-20th century". In AR4, "most of" is defined as more than 50%.
Abbreviations used in the two tables below: ppm = parts-per-million; ppb = parts-per-billion; ppt = parts-per-trillion; W/m2 = watts per square meter
| Gas | Pre-1750 tropospheric concentration |
Recent tropospheric concentration |
Absolute increase since 1750 |
Percentage increase since 1750 |
Increased radiative forcing (W/m2) |
|---|---|---|---|---|---|
| Carbon dioxide (CO2) | 280 ppm | 395.4 ppm | 115.4 ppm | 41.2% | 1.88 |
| Methane (CH 4) |
700 ppb | 1893 ppb / 1762 ppb |
1193 ppb / 1062 ppb |
170.4% / 151.7% |
0.49 |
| Nitrous oxide (N 2O) |
270 ppb | 326 ppb / 324 ppb |
56 ppb / 54 ppb |
20.7% / 20.0% |
0.17 |
| Tropospheric ozone (O 3) |
237 ppb | 337 ppb | 100 ppb | 42% | 0.4 |
| Gas | Recent tropospheric concentration |
Increased radiative forcing (W/m2) |
|---|---|---|
| CFC-11 (trichlorofluoromethane) (CCl 3F) |
236 ppt / 234 ppt |
0.061 |
| CFC-12 (CCl 2F 2) |
527 ppt / 527 ppt |
0.169 |
| CFC-113 (Cl 2FC-CClF 2) |
74 ppt / 74 ppt |
0.022 |
| HCFC-22 (CHClF 2) |
231 ppt / 210 ppt |
0.046 |
| HCFC-141b (CH 3CCl 2F) |
24 ppt / 21 ppt |
0.0036 |
| HCFC-142b (CH 3CClF 2) |
23 ppt / 21 ppt |
0.0042 |
| Halon 1211 (CBrClF 2) |
4.1 ppt / 4.0 ppt |
0.0012 |
| Halon 1301 (CBrClF 3) |
3.3 ppt / 3.3 ppt |
0.001 |
| HFC-134a (CH 2FCF 3) |
75 ppt / 64 ppt |
0.0108 |
| Carbon tetrachloride (CCl 4) |
85 ppt / 83 ppt |
0.0143 |
| Sulfur hexafluoride (SF 6) |
7.79 ppt / 7.39 ppt |
0.0043 |
| Other halocarbons | Varies by substance |
collectively 0.02 |
| Halocarbons in total | 0.3574 |
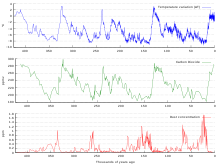
400,000 years of ice core data
Ice cores provide evidence for greenhouse gas concentration variations over the past 800,000 years . Both CO2 and CH
4 vary between glacial and interglacial phases, and concentrations of these gases correlate strongly with temperature. Direct data does not exist for periods earlier than those represented in the ice core record, a record that indicates CO2 mole fractions stayed within a range of 180 ppm to 280 ppm throughout the last 800,000 years, until the increase of the last 250 years. However, various proxies and modeling suggests larger variations in past epochs; 500 million years ago CO2 levels were likely 10 times higher than now. Indeed, higher CO2 concentrations are thought to have prevailed throughout most of the Phanerozoic eon, with concentrations four to six times current concentrations during the Mesozoic era, and ten to fifteen times current concentrations during the early Palaeozoic era until the middle of the Devonian period, about 400 Ma. The spread of land plants is thought to have reduced CO2 concentrations during the late Devonian, and plant activities as both sources and sinks of CO2 have since been important in providing stabilising feedbacks. Earlier still, a 200-million year period of intermittent, widespread glaciation extending close to the equator (Snowball Earth) appears to have been ended suddenly, about 550 Ma, by a colossal volcanic outgassing that raised the CO2 concentration of the atmosphere abruptly to 12%, about 350 times modern levels, causing extreme greenhouse conditions and carbonate deposition as limestone at the rate of about 1 mm per day. This episode marked the close of the Precambrian eon, and was succeeded by the generally warmer conditions of the Phanerozoic, during which multicellular animal and plant life evolved. No volcanic carbon dioxide emission of comparable scale has occurred since. In the modern era, emissions to the atmosphere from volcanoes are approximately 0.645 billion tons of CO2 per year, whereas humans contribute 29 billion tons of CO2 each year.
4 vary between glacial and interglacial phases, and concentrations of these gases correlate strongly with temperature. Direct data does not exist for periods earlier than those represented in the ice core record, a record that indicates CO2 mole fractions stayed within a range of 180 ppm to 280 ppm throughout the last 800,000 years, until the increase of the last 250 years. However, various proxies and modeling suggests larger variations in past epochs; 500 million years ago CO2 levels were likely 10 times higher than now. Indeed, higher CO2 concentrations are thought to have prevailed throughout most of the Phanerozoic eon, with concentrations four to six times current concentrations during the Mesozoic era, and ten to fifteen times current concentrations during the early Palaeozoic era until the middle of the Devonian period, about 400 Ma. The spread of land plants is thought to have reduced CO2 concentrations during the late Devonian, and plant activities as both sources and sinks of CO2 have since been important in providing stabilising feedbacks. Earlier still, a 200-million year period of intermittent, widespread glaciation extending close to the equator (Snowball Earth) appears to have been ended suddenly, about 550 Ma, by a colossal volcanic outgassing that raised the CO2 concentration of the atmosphere abruptly to 12%, about 350 times modern levels, causing extreme greenhouse conditions and carbonate deposition as limestone at the rate of about 1 mm per day. This episode marked the close of the Precambrian eon, and was succeeded by the generally warmer conditions of the Phanerozoic, during which multicellular animal and plant life evolved. No volcanic carbon dioxide emission of comparable scale has occurred since. In the modern era, emissions to the atmosphere from volcanoes are approximately 0.645 billion tons of CO2 per year, whereas humans contribute 29 billion tons of CO2 each year.
Ice cores
Measurements from Antarctic ice cores
show that before industrial emissions started atmospheric CO2 mole fractions were about 280 parts per million (ppm), and stayed between 260 and 280 during the preceding ten thousand years.
Carbon dioxide mole fractions in the atmosphere have gone up by
approximately 35 percent since the 1900s, rising from 280 parts per
million by volume to 387 parts per million in 2009. One study using
evidence from stomata
of fossilized leaves suggests greater variability, with carbon dioxide
mole fractions above 300 ppm during the period seven to ten thousand
years ago, though others have argued that these findings more likely reflect calibration or contamination problems rather than actual CO2 variability.
Because of the way air is trapped in ice (pores in the ice close off
slowly to form bubbles deep within the firn) and the time period
represented in each ice sample analyzed, these figures represent
averages of atmospheric concentrations of up to a few centuries rather
than annual or decadal levels.
Changes since the Industrial Revolution
Recent year-to-year increase of atmospheric CO2.
Major greenhouse gas trends.
Since the beginning of the Industrial Revolution,
the concentrations of most of the greenhouse gases have increased. For
example, the mole fraction of carbon dioxide has increased from 280 ppm
to 400 ppm, or 120 ppm over modern pre-industrial levels. The first
30 ppm increase took place in about 200 years, from the start of the
Industrial Revolution to 1958; however the next 90 ppm increase took
place within 56 years, from 1958 to 2014.
Recent data also shows that the concentration is increasing at a
higher rate. In the 1960s, the average annual increase was only 37% of
what it was in 2000 through 2007.
Today, the stock of carbon in the atmosphere increases by more
than 3 million tonnes per annum (0.04%) compared with the existing
stock.
This increase is the result of human activities by burning fossil
fuels, deforestation and forest degradation in tropical and boreal
regions.
The other greenhouse gases produced from human activity show
similar increases in both amount and rate of increase. Many observations
are available online in a variety of Atmospheric Chemistry Observational Databases.
Anthropogenic greenhouse gases
This graph shows changes in the annual greenhouse gas index (AGGI) between 1979 and 2011. The AGGI measures the levels of greenhouse gases in the atmosphere based on their ability to cause changes in Earth's climate.
This bar graph shows global greenhouse gas emissions by sector from 1990 to 2005, measured in carbon dioxide equivalents.
Since about 1750 human activity has increased the concentration of
carbon dioxide and other greenhouse gases. Measured atmospheric
concentrations of carbon dioxide are currently 100 ppm higher than
pre-industrial levels. Natural sources of carbon dioxide are more than 20 times greater than sources due to human activity,
but over periods longer than a few years natural sources are closely
balanced by natural sinks, mainly photosynthesis of carbon compounds by
plants and marine plankton. As a result of this balance, the atmospheric
mole fraction of carbon dioxide remained between 260 and 280 parts per
million for the 10,000 years between the end of the last glacial maximum
and the start of the industrial era.
It is likely that anthropogenic
(i.e., human-induced) warming, such as that due to elevated greenhouse
gas levels, has had a discernible influence on many physical and
biological systems. Future warming is projected to have a range of impacts, including sea level rise, increased frequencies and severities of some extreme weather events, loss of biodiversity, and regional changes in agricultural productivity.
The main sources of greenhouse gases due to human activity are:
- burning of fossil fuels and deforestation leading to higher carbon dioxide concentrations in the air. Land use change (mainly deforestation in the tropics) account for up to one third of total anthropogenic CO2 emissions.
- livestock enteric fermentation and manure management, paddy rice farming, land use and wetland changes, man-made lakes, pipeline losses, and covered vented landfill emissions leading to higher methane atmospheric concentrations. Many of the newer style fully vented septic systems that enhance and target the fermentation process also are sources of atmospheric methane.
- use of chlorofluorocarbons (CFCs) in refrigeration systems, and use of CFCs and halons in fire suppression systems and manufacturing processes.
- agricultural activities, including the use of fertilizers, that lead to higher nitrous oxide (N
2O) concentrations.
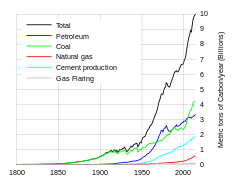
The seven sources of CO2 from fossil fuel combustion are (with percentage contributions for 2000–2004):
| Seven main fossil fuel combustion sources |
Contribution (%) |
|---|---|
| Liquid fuels (e.g., gasoline, fuel oil) | 36% |
| Solid fuels (e.g., coal) | 35% |
| Gaseous fuels (e.g., natural gas) | 20% |
| Cement production | 3 % |
| Flaring gas industrially and at wells | < 1% |
| Non-fuel hydrocarbons | < 1% |
| "International bunker fuels" of transport not included in national inventories |
4 % |
Carbon dioxide, methane, nitrous oxide (N
2O) and three groups of fluorinated gases (sulfur hexafluoride (SF
6), hydrofluorocarbons (HFCs), and perfluorocarbons (PFCs)) are the major anthropogenic greenhouse gases, and are regulated under the Kyoto Protocol international treaty, which came into force in 2005. Emissions limitations specified in the Kyoto Protocol expired in 2012. The Cancún agreement, agreed on in 2010, includes voluntary pledges made by 76 countries to control emissions. At the time of the agreement, these 76 countries were collectively responsible for 85% of annual global emissions.
2O) and three groups of fluorinated gases (sulfur hexafluoride (SF
6), hydrofluorocarbons (HFCs), and perfluorocarbons (PFCs)) are the major anthropogenic greenhouse gases, and are regulated under the Kyoto Protocol international treaty, which came into force in 2005. Emissions limitations specified in the Kyoto Protocol expired in 2012. The Cancún agreement, agreed on in 2010, includes voluntary pledges made by 76 countries to control emissions. At the time of the agreement, these 76 countries were collectively responsible for 85% of annual global emissions.
Although CFCs are greenhouse gases, they are regulated by the Montreal Protocol, which was motivated by CFCs' contribution to ozone depletion
rather than by their contribution to global warming. Note that ozone
depletion has only a minor role in greenhouse warming, though the two
processes often are confused in the media. On 15 October 2016,
negotiators from over 170 nations meeting at the summit of the United Nations Environment Programme reached a legally binding accord to phase out hydrofluorocarbons (HFCs) in an amendment to the Montreal Protocol.
Sectors
This
figure shows the relative fraction of anthropogenic greenhouse gases
coming from each of eight categories of sources, as estimated by the
Emission Database for Global Atmospheric Research version 3.2, fast
track 2000 project [1]. These values are intended to provide a snapshot
of global annual greenhouse gas emissions in the year 2000. The top
panel shows the sum over all anthropogenic greenhouse gases, weighted by
their global warming potential over the next 100 years. This consists
of 72% carbon dioxide, 18% methane, 8% nitrous oxide and 1% other gases.
Lower panels show the comparable information for each of these three
primary greenhouse gases, with the same coloring of sectors as used in
the top chart. Segments with less than 1% fraction are not labeled.
Tourism
According to UNEP global tourism is closely linked to climate change.
Tourism is a significant contributor to the increasing concentrations
of greenhouse gases in the atmosphere. Tourism accounts for about 50% of
traffic movements. Rapidly expanding air traffic contributes about 2.5%
of the production of CO2. The number of international
travelers is expected to increase from 594 million in 1996 to 1.6
billion by 2020, adding greatly to the problem unless steps are taken to
reduce emissions.
Trucking and haulage
The trucking and haulage industry plays a part in production of CO2,
contributing around 20% of the UK's total carbon emissions a year, with
only the energy industry having a larger impact at around 39%.
Average carbon emissions within the haulage industry are falling—in the
thirty-year period from 1977 to 2007, the carbon emissions associated
with a 200-mile journey fell by 21 percent; NOx emissions are also down
87 percent, whereas journey times have fallen by around a third. Due to their size, HGVs
often receive criticism regarding their CO2 emissions; however, rapid
development in engine technology and fuel management is having a largely
positive effect.
Plastic
Plastic is produced mainly from Fossil fuels.
Plastic manufacturing is estimated to use 8 percent of yearly global
oil production. The EPA estimates as many as five ounces of carbon
dioxide are emitted for each ounce of polyethylene terephthalate (PET) produced—the type of plastic most commonly used for beverage bottles, the transportation produce greenhouse gases also.
Plastic waste emits carbon dioxide when it degrades. In 2018 research
claimed that some of the most common plastics in the environment release
the greenhouse gases Methane and Ethylene when exposed to sunlight in an amount that can affect the earth climate.
From the other side, if it is placed in a landfill, it becomes a carbon sink although biodegradable plastics have caused methane emissions. Due to the lightness of plastic versus glass or metal, plastic may
reduce energy consumption. For example, packaging beverages in PET
plastic rather than glass or metal is estimated to save 52% in
transportation energy, if the glass or metal packag is single use, of
course .
Role of water vapor
Increasing water vapor in the stratosphere at Boulder, Colorado.
Water vapor
accounts for the largest percentage of the greenhouse effect, between
36% and 66% for clear sky conditions and between 66% and 85% when
including clouds.
Water vapor concentrations fluctuate regionally, but human activity
does not directly affect water vapor concentrations except at local
scales, such as near irrigated fields. Indirectly, human activity that
increases global temperatures will increase water vapor concentrations, a
process known as water vapor feedback.
The atmospheric concentration of vapor is highly variable and depends
largely on temperature, from less than 0.01% in extremely cold regions
up to 3% by mass in saturated air at about 32 °C.
The average residence time of a water molecule in the atmosphere
is only about nine days, compared to years or centuries for other
greenhouse gases such as CH
4 and CO2. Thus, water vapor responds to and amplifies effects of the other greenhouse gases. The Clausius–Clapeyron relation establishes that more water vapor will be present per unit volume at elevated temperatures. This and other basic principles indicate that warming associated with increased concentrations of the other greenhouse gases also will increase the concentration of water vapor (assuming that the relative humidity remains approximately constant; modeling and observational studies find that this is indeed so). Because water vapor is a greenhouse gas, this results in further warming and so is a "positive feedback" that amplifies the original warming. Eventually other earth processes offset these positive feedbacks, stabilizing the global temperature at a new equilibrium and preventing the loss of Earth's water through a Venus-like runaway greenhouse effect.
4 and CO2. Thus, water vapor responds to and amplifies effects of the other greenhouse gases. The Clausius–Clapeyron relation establishes that more water vapor will be present per unit volume at elevated temperatures. This and other basic principles indicate that warming associated with increased concentrations of the other greenhouse gases also will increase the concentration of water vapor (assuming that the relative humidity remains approximately constant; modeling and observational studies find that this is indeed so). Because water vapor is a greenhouse gas, this results in further warming and so is a "positive feedback" that amplifies the original warming. Eventually other earth processes offset these positive feedbacks, stabilizing the global temperature at a new equilibrium and preventing the loss of Earth's water through a Venus-like runaway greenhouse effect.
Direct greenhouse gas emissions
Between the period 1970 to 2004, greenhouse gas emissions (measured in CO2-equivalent) increased at an average rate of 1.6% per year, with CO2 emissions from the use of fossil fuels growing at a rate of 1.9% per year. Total anthropogenic emissions at the end of 2009 were estimated at 49.5 gigatonnes CO2-equivalent. These emissions include CO2
from fossil fuel use and from land use, as well as emissions of
methane, nitrous oxide and other greenhouse gases covered by the Kyoto Protocol.
At present, the primary source of CO2 emissions is the burning of coal, natural gas, and petroleum for electricity and heat.
Regional and national attribution of emissions
According to the Environmental Protection Agency (EPA), GHG emissions in the United States can be traced from different sectors.
There are several different ways of measuring greenhouse gas emissions, for example, see World Bank (2010) for tables of national emissions data. Some variables that have been reported include:
- Definition of measurement boundaries: Emissions can be attributed geographically, to the area where they were emitted (the territory principle) or by the activity principle to the territory produced the emissions. These two principles result in different totals when measuring, for example, electricity importation from one country to another, or emissions at an international airport.
- Time horizon of different gases: Contribution of a given greenhouse gas is reported as a CO2 equivalent. The calculation to determine this takes into account how long that gas remains in the atmosphere. This is not always known accurately and calculations must be regularly updated to reflect new information.
- What sectors are included in the calculation (e.g., energy industries, industrial processes, agriculture etc.): There is often a conflict between transparency and availability of data.
- The measurement protocol itself: This may be via direct measurement or estimation. The four main methods are the emission factor-based method, mass balance method, predictive emissions monitoring systems, and continuous emissions monitoring systems. These methods differ in accuracy, cost, and usability.
These different measures are sometimes used by different countries to
assert various policy/ethical positions on climate change (Banuri et al., 1996, p. 94).
The use of different measures leads to a lack of comparability, which is
problematic when monitoring progress towards targets. There are
arguments for the adoption of a common measurement tool, or at least the
development of communication between different tools.
Emissions may be measured over long time periods. This
measurement type is called historical or cumulative emissions.
Cumulative emissions give some indication of who is responsible for the
build-up in the atmospheric concentration of greenhouse gases (IEA,
2007, p. 199).
The national accounts balance would be positively related to
carbon emissions. The national accounts balance shows the difference
between exports and imports. For many richer nations, such as the United
States, the accounts balance is negative because more goods are
imported than they are exported. This is mostly due to the fact that it
is cheaper to produce goods outside of developed countries, leading the
economies of developed countries to become increasingly dependent on
services and not goods. We believed that a positive accounts balance
would means that more production was occurring in a country, so more
factories working would increase carbon emission levels.
Emissions may also be measured across shorter time periods.
Emissions changes may, for example, be measured against a base year of
1990. 1990 was used in the United Nations Framework Convention on Climate Change (UNFCCC) as the base year for emissions, and is also used in the Kyoto Protocol (some gases are also measured from the year 1995). A country's emissions may also be reported as a proportion of global emissions for a particular year.
Another measurement is of per capita emissions. This divides a country's total annual emissions by its mid-year population. Per capita emissions may be based on historical or annual emissions (Banuri et al., 1996, pp. 106–07).
While cities are sometimes considered to be disproportionate
contributors to emissions, per-capita emissions tend to be lower for
cities than the averages in their countries.
From land-use change
Greenhouse gas emissions from agriculture, forestry and other land use, 1970–2010.
Land-use change, e.g., the clearing of forests for agricultural use,
can affect the concentration of greenhouse gases in the atmosphere by
altering how much carbon flows out of the atmosphere into carbon sinks.
Accounting for land-use change can be understood as an attempt to
measure "net" emissions, i.e., gross emissions from all sources minus
the removal of emissions from the atmosphere by carbon sinks (Banuri et al., 1996, pp. 92–93).
There are substantial uncertainties in the measurement of net carbon emissions. Additionally, there is controversy over how carbon sinks should be allocated between different regions and over time (Banuri et al., 1996, p. 93).
For instance, concentrating on more recent changes in carbon sinks is
likely to favour those regions that have deforested earlier, e.g.,
Europe.
Greenhouse gas intensity
Greenhouse gas intensity in the year 2000, including land-use change.
|
Carbon intensity of GDP (using PPP) for different regions, 1982–2011.
|
Carbon intensity of GDP (using MER) for different regions, 1982–2011.
|
Greenhouse gas intensity is a ratio between greenhouse gas emissions
and another metric, e.g., gross domestic product (GDP) or energy use.
The terms "carbon intensity" and "emissions intensity" are also sometimes used. Emission intensities may be calculated using market exchange rates (MER) or purchasing power parity (PPP) (Banuri et al., 1996, p. 96).
Calculations based on MER show large differences in intensities between
developed and developing countries, whereas calculations based on PPP
show smaller differences.
Cumulative and historical emissions
Cumulative energy-related CO2 emissions between the years 1850–2005 grouped into low-income, middle-income, high-income, the EU-15, and the OECD countries.
Map of cumulative per capita anthropogenic atmospheric CO2 emissions by country. Cumulative emissions include land use change, and are measured between the years 1950 and 2000.
Cumulative anthropogenic (i.e., human-emitted) emissions of CO2 from fossil fuel use are a major cause of global warming, and give some indication of which countries have contributed most to human-induced climate change.
| Region | Industrial CO2 |
Total CO2 |
|---|---|---|
| OECD North America | 33.2 | 29.7 |
| OECD Europe | 26.1 | 16.6 |
| Former USSR | 14.1 | 12.5 |
| China | 5.5 | 6.0 |
| Eastern Europe | 5.5 | 4.8 |
The table above to the left is based on Banuri et al. (1996, p. 94). Overall, developed countries accounted for 83.8% of industrial CO2 emissions over this time period, and 67.8% of total CO2 emissions. Developing countries accounted for industrial CO2 emissions of 16.2% over this time period, and 32.2% of total CO2 emissions. The estimate of total CO2 emissions includes biotic carbon emissions, mainly from deforestation. Banuri et al. (1996, p. 94)
calculated per capita cumulative emissions based on then-current
population. The ratio in per capita emissions between industrialized
countries and developing countries was estimated at more than 10 to 1.
Including biotic emissions brings about the same controversy
mentioned earlier regarding carbon sinks and land-use change (Banuri et al., 1996, pp. 93–94).
The actual calculation of net emissions is very complex, and is
affected by how carbon sinks are allocated between regions and the
dynamics of the climate system.
Non-OECD countries accounted for 42% of cumulative energy-related CO2 emissions between 1890 and 2007.
Over this time period, the US accounted for 28% of emissions; the EU,
23%; Russia, 11%; China, 9%; other OECD countries, 5%; Japan, 4%; India,
3%; and the rest of the world, 18%.
Changes since a particular base year
Between 1970 and 2004, global growth in annual CO2 emissions was driven by North America, Asia, and the Middle East. The sharp acceleration in CO2
emissions since 2000 to more than a 3% increase per year (more than
2 ppm per year) from 1.1% per year during the 1990s is attributable to
the lapse of formerly declining trends in carbon intensity
of both developing and developed nations. China was responsible for
most of global growth in emissions during this period. Localized
plummeting emissions associated with the collapse of the Soviet Union
have been followed by slow emissions growth in this region due to more efficient energy use, made necessary by the increasing proportion of it that is exported. In comparison, methane has not increased appreciably, and N
2O by 0.25% y−1.
2O by 0.25% y−1.
Using different base years for measuring emissions has an effect on estimates of national contributions to global warming.
This can be calculated by dividing a country's highest contribution to
global warming starting from a particular base year, by that country's
minimum contribution to global warming starting from a particular base
year. Choosing between different base years of 1750, 1900, 1950, and
1990 has a significant effect for most countries. Within the G8 group of countries, it is most significant for the UK, France and Germany. These countries have a long history of CO2 emissions.
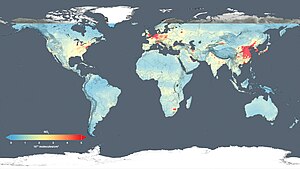
Annual emissions
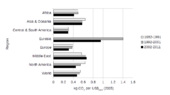
Per capita anthropogenic greenhouse gas emissions by country for the year 2000 including land-use change.
Annual per capita emissions in the industrialized countries are
typically as much as ten times the average in developing countries. Due to China's fast economic development, its annual per capita emissions are quickly approaching the levels of those in the Annex I group of the Kyoto Protocol (i.e., the developed countries excluding the US). Other countries with fast growing emissions are South Korea,
Iran, and Australia (which apart from the oil rich Persian Gulf states,
now has the highest per capita emission rate in the world). On the other
hand, annual per capita emissions of the EU-15 and the US are gradually
decreasing over time. Emissions in Russia and Ukraine have decreased fastest since 1990 due to economic restructuring in these countries.
Energy statistics for fast growing economies are less accurate
than those for the industrialized countries. For China's annual
emissions in 2008, the Netherlands Environmental Assessment Agency estimated an uncertainty range of about 10%.
The greenhouse gas footprint refers to the emissions resulting from the creation of products or services. It is more comprehensive than the commonly used carbon footprint, which measures only carbon dioxide, one of many greenhouse gases.
2015 was the first year to see both total global economic growth and a reduction of carbon emissions.
Top emitter countries
Global carbon dioxide emissions by country.
The
top 40 countries emitting all greenhouse gases, showing both that
derived from all sources including land clearance and forestry and also
the CO2 component excluding those sources. Per capita figures are
included. "World Resources Institute data".. Note that Indonesia and Brazil show very much higher than on graphs simply showing fossil fuel use.
Annual
In 2009, the annual top ten emitting countries accounted for about two-thirds of the world's annual energy-related CO2 emissions.
| Country | % of global total annual emissions |
Tonnes of GHG per capita |
|---|---|---|
| 23.6 | 5.1 | |
| 17.9 | 16.9 | |
| 5.5 | 1.4 | |
| 5.3 | 10.8 | |
| 3.8 | 8.6 | |
| 2.6 | 9.2 | |
| 1.8 | 7.3 | |
| 1.8 | 15.4 | |
| 1.8 | 10.6 | |
| 1.6 | 7.5 |
Cumulative
| Country | % of world total |
Metric tonnes CO2 per person |
|---|---|---|
| 28.5 | 1,132.7 | |
| 9.36 | 85.4 | |
| 7.95 | 677.2 | |
| 6.78 | 998.9 | |
| 5.73 | 1,127.8 | |
| 3.88 | 367 | |
| 2.73 | 514.9 | |
| 2.52 | 26.7 | |
| 2.17 | 789.2 | |
| 2.13 | 556.4 |
Embedded emissions
One way of attributing greenhouse gas (GHG) emissions is to measure the embedded emissions
(also referred to as "embodied emissions") of goods that are being
consumed. Emissions are usually measured according to production, rather
than consumption. For example, in the main international treaty on climate change (the UNFCCC), countries report on emissions produced within their borders, e.g., the emissions produced from burning fossil fuels.
Under a production-based accounting of emissions, embedded emissions on
imported goods are attributed to the exporting, rather than the
importing, country. Under a consumption-based accounting of emissions,
embedded emissions on imported goods are attributed to the importing
country, rather than the exporting, country.
Davis and Caldeira (2010) found that a substantial proportion of CO2
emissions are traded internationally. The net effect of trade was to
export emissions from China and other emerging markets to consumers in
the US, Japan, and Western Europe. Based on annual emissions data from
the year 2004, and on a per-capita consumption basis, the top-5 emitting
countries were found to be (in tCO2 per person, per year): Luxembourg (34.7), the US (22.0), Singapore (20.2), Australia (16.7), and Canada (16.6). Carbon Trust research revealed that approximately 25% of all CO2
emissions from human activities 'flow' (i.e., are imported or exported)
from one country to another. Major developed economies were found to be
typically net importers of embodied carbon emissions—with UK
consumption emissions 34% higher than production emissions, and Germany
(29%), Japan (19%) and the US (13%) also significant net importers of
embodied emissions.
Effect of policy
Governments have taken action to reduce greenhouse gas emissions (climate change mitigation). Assessments of policy effectiveness have included work by the Intergovernmental Panel on Climate Change, International Energy Agency, and United Nations Environment Programme. Policies implemented by governments have included national and regional targets to reduce emissions, promoting energy efficiency, and support for renewable energy
such as Solar energy as an effective use of renewable energy because
solar uses energy from the sun and does not release pollutants into the
air.
Countries and regions listed in Annex I of the United Nations Framework Convention on Climate Change
(UNFCCC) (i.e., the OECD and former planned economies of the Soviet
Union) are required to submit periodic assessments to the UNFCCC of
actions they are taking to address climate change. Analysis by the UNFCCC (2011) suggested that policies and measures undertaken by Annex I Parties may have produced emission savings of 1.5 thousand Tg CO2-eq in the year 2010, with most savings made in the energy sector. The projected emissions saving of 1.5 thousand Tg CO2-eq is measured against a hypothetical "baseline"
of Annex I emissions, i.e., projected Annex I emissions in the absence
of policies and measures. The total projected Annex I saving of 1.5
thousand CO2-eq does not include emissions savings in seven of the Annex I Parties.
Projections
A wide range of projections of future emissions have been produced. Rogner et al. (2007) assessed the scientific literature on greenhouse gas projections. Rogner et al. (2007)
concluded that unless energy policies changed substantially, the world
would continue to depend on fossil fuels until 2025–2030. Projections
suggest that more than 80% of the world's energy will come from fossil
fuels. This conclusion was based on "much evidence" and "high agreement"
in the literature. Projected annual energy-related CO2 emissions in 2030 were 40–110% higher than in 2000, with two-thirds of the increase originating in developing countries. Projected annual per capita emissions in developed country regions remained substantially lower (2.8–5.1 tonnes CO2) than those in developed country regions (9.6–15.1 tonnes CO2). Projections consistently showed increase in annual world emissions of "Kyoto" gases, measured in CO2-equivalent) of 25–90% by 2030, compared to 2000.
Relative CO2 emission from various fuels
One liter of gasoline, when used as a fuel, produces 2.32 kg
(about 1300 liters or 1.3 cubic meters) of carbon dioxide, a greenhouse
gas. One US gallon produces 19.4 lb (1,291.5 gallons or 172.65 cubic
feet)
| Fuel name | CO2 emitted (lbs/106 Btu) |
CO2 emitted (g/MJ) |
CO2 emitted (g/kWh) |
|---|---|---|---|
| Natural gas | 117 | 50.30 | 181.08 |
| Liquefied petroleum gas | 139 | 59.76 | 215.14 |
| Propane | 139 | 59.76 | 215.14 |
| Aviation gasoline | 153 | 65.78 | 236.81 |
| Automobile gasoline | 156 | 67.07 | 241.45 |
| Kerosene | 159 | 68.36 | 246.10 |
| Fuel oil | 161 | 69.22 | 249.19 |
| Tires/tire derived fuel | 189 | 81.26 | 292.54 |
| Wood and wood waste | 195 | 83.83 | 301.79 |
| Coal (bituminous) | 205 | 88.13 | 317.27 |
| Coal (sub-bituminous) | 213 | 91.57 | 329.65 |
| Coal (lignite) | 215 | 92.43 | 332.75 |
| Petroleum coke | 225 | 96.73 | 348.23 |
| Tar-sand Bitumen |
|
|
|
| Coal (anthracite) | 227 | 97.59 | 351.32 |
Life-cycle greenhouse-gas emissions of energy sources
A literature review of numerous energy sources CO2 emissions by the IPCC in 2011, found that, the CO2 emission value that fell within the 50th percentile of all total life cycle emissions studies conducted was as follows.
| Technology | Description | 50th percentile (g CO2/kWhe) |
|---|---|---|
| Hydroelectric | reservoir | 4 |
| Ocean Energy | wave and tidal | 8 |
| Wind | onshore | 12 |
| Nuclear | various generation II reactor types | 16 |
| Biomass | various | 18 |
| Solar thermal | parabolic trough | 22 |
| Geothermal | hot dry rock | 45 |
| Solar PV | Polycrystalline silicon | 46 |
| Natural gas | various combined cycle turbines without scrubbing | 469 |
| Coal | various generator types without scrubbing | 1001 |
Removal from the atmosphere ("sinks")
Natural processes
Greenhouse gases can be removed from the atmosphere by various processes, as a consequence of:
- a physical change (condensation and precipitation remove water vapor from the atmosphere).
- a chemical reaction within the atmosphere. For example, methane is oxidized by reaction with naturally occurring hydroxyl radical, OH· and degraded to CO2 and water vapor (CO2 from the oxidation of methane is not included in the methane Global warming potential). Other chemical reactions include solution and solid phase chemistry occurring in atmospheric aerosols.
- a physical exchange between the atmosphere and the other compartments of the planet. An example is the mixing of atmospheric gases into the oceans.
- a chemical change at the interface between the atmosphere and the other compartments of the planet. This is the case for CO2, which is reduced by photosynthesis of plants, and which, after dissolving in the oceans, reacts to form carbonic acid and bicarbonate and carbonate ions (see ocean acidification).
- a photochemical change. Halocarbons are dissociated by UV light releasing Cl· and F· as free radicals in the stratosphere with harmful effects on ozone (halocarbons are generally too stable to disappear by chemical reaction in the atmosphere).
Negative emissions
A number of technologies remove greenhouse gases emissions from the
atmosphere. Most widely analysed are those that remove carbon dioxide
from the atmosphere, either to geologic formations such as bio-energy with carbon capture and storage and carbon dioxide air capture, or to the soil as in the case with biochar.
The IPCC has pointed out that many long-term climate scenario models
require large-scale manmade negative emissions to avoid serious climate
change.
History of scientific research
In the late 19th century scientists experimentally discovered that N2 and O2
do not absorb infrared radiation (called, at that time, "dark
radiation"), while water (both as true vapor and condensed in the form
of microscopic droplets suspended in clouds) and CO2 and
other poly-atomic gaseous molecules do absorb infrared radiation. In the
early 20th century researchers realized that greenhouse gases in the
atmosphere made Earth's overall temperature higher than it would be
without them. During the late 20th century, a scientific consensus
evolved that increasing concentrations of greenhouse gases in the
atmosphere cause a substantial rise in global temperatures and changes
to other parts of the climate system, with consequences for the environment and for human health.