| Hepatitis C | |
|---|---|
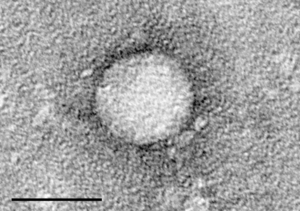 | |
| Electron micrograph of hepatitis C virus from cell culture (scale = 50 nanometers) | |
| Specialty | Gastroenterology, Infectious disease |
| Symptoms | Typically none |
| Complications | Liver failure, liver cancer, esophageal and gastric varices |
| Duration | Long term (80%) |
| Causes | Hepatitis C virus usually spread by blood-to-blood contact |
| Diagnostic method | Blood testing for antibodies or viral RNA |
| Prevention | Clean needles, testing donated blood |
| Treatment | Medications, liver transplant |
| Medication | Sofosbuvir, simeprevir |
| Frequency | 143 million / 2% (2015) |
| Deaths | 496,000 (2015) |
Hepatitis C is an infectious disease caused by the hepatitis C virus (HCV) that primarily affects the liver. During the initial infection people often have mild or no symptoms. Occasionally a fever, dark urine, abdominal pain, and yellow tinged skin occurs. The virus persists in the liver in about 75% to 85% of those initially infected. Early on chronic infection typically has no symptoms. Over many years however, it often leads to liver disease and occasionally cirrhosis. In some cases, those with cirrhosis will develop serious complications such as liver failure, liver cancer, or dilated blood vessels in the esophagus and stomach.
HCV is spread primarily by blood-to-blood contact associated with intravenous drug use, poorly sterilized medical equipment, needlestick injuries in healthcare, and transfusions. Using blood screening, the risk from a transfusion is less than one per two million. It may also be spread from an infected mother to her baby during birth. It is not spread by superficial contact. It is one of five known hepatitis viruses: A, B, C, D, and E. Diagnosis is by blood testing to look for either antibodies to the virus or its RNA. Testing is recommended in all people who are at risk.
There is no vaccine against hepatitis C. Prevention includes harm reduction efforts among people who use intravenous drugs and testing donated blood. Chronic infection can be cured about 95% of the time with antiviral medications such as sofosbuvir or simeprevir. Peginterferon and ribavirin were earlier generation treatments that had a cure rate of less than 50% and greater side effects. Getting access to the newer treatments however can be expensive. Those who develop cirrhosis or liver cancer may require a liver transplant. Hepatitis C is the leading reason for liver transplantation, though the virus usually recurs after transplantation.
An estimated 143 million people (2%) worldwide are infected with hepatitis C as of 2015. In 2013 about 11 million new cases occurred. It occurs most commonly in Africa and Central and East Asia. About 167,000 deaths due to liver cancer and 326,000 deaths due to cirrhosis occurred in 2015 due to hepatitis C. The existence of hepatitis C – originally identifiable only as a type of non-A non-B hepatitis – was suggested in the 1970s and proven in 1989. Hepatitis C infects only humans and chimpanzees.
Signs and symptoms
Acute infection
Hepatitis C infection causes acute symptoms in 15% of cases. Symptoms are generally mild and vague, including a decreased appetite, fatigue, nausea, muscle or joint pains, and weight loss and rarely does acute liver failure result. Most cases of acute infection are not associated with jaundice. The infection resolves spontaneously in 10–50% of cases, which occurs more frequently in individuals who are young and female.
Chronic infection
About 80% of those exposed to the virus develop a chronic infection.
This is defined as the presence of detectable viral replication for at
least six months. Most experience minimal or no symptoms during the
initial few decades of the infection. Chronic hepatitis C can be associated with fatigue and mild cognitive problems. Chronic infection after several years may cause cirrhosis or liver cancer. The liver enzymes are normal in 7–53%. Late relapses after apparent cure have been reported, but these can be difficult to distinguish from reinfection.
Fatty changes to the liver occur in about half of those infected and are usually present before cirrhosis develops. Usually (80% of the time) this change affects less than a third of the liver. Worldwide hepatitis C is the cause of 27% of cirrhosis cases and 25% of hepatocellular carcinoma. About 10–30% of those infected develop cirrhosis over 30 years. Cirrhosis is more common in those also infected with hepatitis B, schistosoma, or HIV, in alcoholics and in those of male gender. In those with hepatitis C, excess alcohol increases the risk of developing cirrhosis 100-fold. Those who develop cirrhosis have a 20-fold greater risk of hepatocellular carcinoma. This transformation occurs at a rate of 1–3% per year. Being infected with hepatitis B in addition to hepatitis C increases this risk further.
Liver cirrhosis may lead to portal hypertension, ascites (accumulation of fluid in the abdomen), easy bruising or bleeding, varices (enlarged veins, especially in the stomach and esophagus), jaundice, and a syndrome of cognitive impairment known as hepatic encephalopathy. Ascites occurs at some stage in more than half of those who have a chronic infection.
Extrahepatic complications
The most common problem due to hepatitis C but not involving the liver is mixed cryoglobulinemia (usually the type II form) — an inflammation of small and medium-sized blood vessels. Hepatitis C is also associated with the autoimmune disorder such as Sjögren's syndrome, lichen planus, a low platelet count, porphyria cutanea tarda, necrolytic acral erythema, insulin resistance, diabetes mellitus, diabetic nephropathy, autoimmune thyroiditis, and B-cell lymphoproliferative disorders. 20–30% of people infected have rheumatoid factor — a type of antibody. Possible associations include Hyde's prurigo nodularis and membranoproliferative glomerulonephritis. Cardiomyopathy with associated abnormal heart rhythms has also been reported. A variety of central nervous system disorders has been reported. Chronic infection seems to be associated with an increased risk of pancreatic cancer. People may experience other issues in the mouth such as dryness, salivary duct stones, and crusted lesions around the mouth.
Occult infection
Persons who have been infected with hepatitis C may appear to clear the virus but remain infected. The virus is not detectable with conventional testing but can be found with ultra-sensitive tests. The original method of detection was by demonstrating the viral genome
within liver biopsies, but newer methods include an antibody test for
the virus' core protein and the detection of the viral genome after
first concentrating the viral particles by ultracentrifugation.
A form of infection with persistently moderately elevated serum liver
enzymes but without antibodies to hepatitis C has also been reported. This form is known as cryptogenic occult infection.
Several clinical pictures have been associated with this type of infection.
It may be found in people with anti-hepatitis-C antibodies but with
normal serum levels of liver enzymes; in antibody-negative people with
ongoing elevated liver enzymes of unknown cause; in healthy populations
without evidence of liver disease; and in groups at risk for HCV
infection including those on hemodialysis or family members of people
with occult HCV. The clinical relevance of this form of infection is
under investigation.
The consequences of occult infection appear to be less severe than with
chronic infection but can vary from minimal to hepatocellular
carcinoma.
The rate of occult infection in those apparently cured is controversial but appears to be low.
40% of those with hepatitis but with both negative hepatitis C serology
and the absence of detectable viral genome in the serum have hepatitis C
virus in the liver on biopsy. How commonly this occurs in children is unknown.
Virology
The hepatitis C virus (HCV) is a small, enveloped, single-stranded, positive-sense RNA virus. It is a member of the genus Hepacivirus in the family Flaviviridae. There are seven major genotypes of HCV, which are known as genotypes one to seven.
The genotypes are divided into several subtypes with the number of
subtypes depending on the genotype. In the United States, about 70% of
cases are caused by genotype 1, 20% by genotype 2 and about 1% by each
of the other genotypes. Genotype 1 is also the most common in South America and Europe.
The half life of the virus particles in the serum is around 3 hours and may be as short as 45 minutes. In an infected person, about 1012 virus particles are produced each day. In addition to replicating in the liver the virus can multiply in lymphocytes.
Transmission
Hepatitis C infection in the United States by source
The primary route of transmission in the developed world is intravenous drug use (IDU), while in the developing world the main methods are blood transfusions and unsafe medical procedures. The cause of transmission remains unknown in 20% of cases; however, many of these are believed to be accounted for by IDU.
Drug use
Intravenous drug use (IDU) is a major risk factor for hepatitis C in many parts of the world. Of 77 countries reviewed, 25 (including the United States) were found to have prevalences of hepatitis C in the intravenous drug user population of between 60% and 80%. Twelve countries had rates greater than 80%. It is believed that ten million intravenous drug users are infected with hepatitis C; China (1.6 million), the United States (1.5 million), and Russia (1.3 million) have the highest absolute totals. Occurrence of hepatitis C among prison inmates in the United States
is 10 to 20 times that of the occurrence observed in the general
population; this has been attributed to high-risk behavior in prisons
such as IDU and tattooing with nonsterile equipment. Shared intranasal drug use may also be a risk factor.
Healthcare exposure
Blood transfusion, transfusion of blood products, or organ transplants without HCV screening carry significant risks of infection. The United States instituted universal screening in 1992 and Canada instituted universal screening in 1990. This decreased the risk from one in 200 units to between one in 10,000 to one in 10,000,000 per unit of blood. This low risk remains as there is a period of about 11–70 days between the potential blood donor's acquiring hepatitis C and the blood's testing positive depending on the method. Some countries do not screen for hepatitis C due to the cost.
Those who have experienced a needle stick injury from someone who was HCV positive have about a 1.8% chance of subsequently contracting the disease themselves. The risk is greater if the needle in question is hollow and the puncture wound is deep.
There is a risk from mucosal exposures to blood, but this risk is low,
and there is no risk if blood exposure occurs on intact skin.
Hospital equipment has also been documented as a method of transmission of hepatitis C,
including reuse of needles and syringes; multiple-use medication vials;
infusion bags; and improperly sterilized surgical equipment, among
others.
Limitations in the implementation and enforcement of stringent standard
precautions in public and private medical and dental facilities are
known to be the primary cause of the spread of HCV in Egypt, the country with highest rate of infection in the world.
Sexual intercourse
Sexual transmission of hepatitis C is uncommon. Studies examining the risk of HCV transmission between heterosexual partners, when one is infected and the other is not, have found very low risks. Sexual practices that involve higher levels of trauma to the anogenital mucosa, such as anal penetrative sex, or that occur when there is a concurrent sexually transmitted infection, including HIV or genital ulceration, present greater risks. The United States Department of Veterans Affairs recommends condom use to prevent hepatitis C transmission in those with multiple partners, but not those in relationships that involve only a single partner.
Body modification
Tattooing is associated with two to threefold increased risk of hepatitis C. This can be due to either improperly sterilized equipment or contamination of the dyes being used. Tattoos or piercings
performed either before the mid-1980s, "underground," or
nonprofessionally are of particular concern, since sterile techniques in
such settings may be lacking. The risk also appears to be greater for
larger tattoos. It is estimated that nearly half of prison inmates share unsterilized tattooing equipment. It is rare for tattoos in a licensed facility to be directly associated with HCV infection.
Personal-care items such as razors, toothbrushes, and manicuring or
pedicuring equipment can be contaminated with blood. Sharing such items
can potentially lead to exposure to HCV. Appropriate caution should be taken regarding any medical condition that results in bleeding, such as cuts and sores. HCV is not spread through casual contact, such as hugging, kissing, or sharing eating or cooking utensils. Neither is it transmitted through food or water.
Mother-to-child transmission
Mother-to-child transmission of hepatitis C occurs in less than 10% of pregnancies. There are no measures that alter this risk. It is not clear when transmission occurs during pregnancy, but it may occur both during gestation and at delivery. A long labor is associated with a greater risk of transmission. There is no evidence that breastfeeding
spreads HCV; however, to be cautious, an infected mother is advised to
avoid breastfeeding if her nipples are cracked and bleeding, or if her viral loads are high.
Diagnosis
Serologic profile of Hepatitis C infection
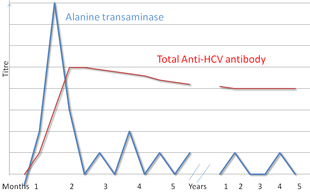
There are a number of diagnostic tests for hepatitis C, including HCV antibody enzyme immunoassay or ELISA, recombinant immunoblot assay, and quantitative HCV RNA polymerase chain reaction (PCR). HCV RNA
can be detected by PCR typically one to two weeks after infection,
while antibodies can take substantially longer to form and thus be
detected.
Chronic hepatitis C is defined as infection with the hepatitis C virus persisting for more than six months based on the presence of its RNA. Chronic infections are typically asymptomatic during the first few decades, and thus are most commonly discovered following the investigation of elevated liver enzyme levels
or during a routine screening of high-risk individuals. Testing is not
able to distinguish between acute and chronic infections. Diagnosis in the infant is difficult as maternal antibodies may persist for up to 18 months.
Serology
Hepatitis C testing typically begins with blood testing to detect the presence of antibodies to the HCV, using an enzyme immunoassay. If this test is positive, a confirmatory test is then performed to verify the immunoassay and to determine the viral load.
A recombinant immunoblot assay is used to verify the immunoassay and
the viral load is determined by an HCV RNA polymerase chain reaction.
If there is no RNA and the immunoblot is positive, it means that the
person tested had a previous infection but cleared it either with
treatment or spontaneously; if the immunoblot is negative, it means that
the immunoassay was wrong. It takes about 6–8 weeks following infection before the immunoassay will test positive. A number of tests are available as point of care testing which means that results are available within 30 minutes.
Liver enzymes are variable during the initial part of the infection and on average begin to rise at seven weeks after infection. The elevation of liver enzymes does not closely follow disease severity.
There are reports of negative plasma PCR for the viral genome
with positive PCR for the viral genome within peripheral blood monocytes
of liver cells. This condition has been termed occult HCV infection and it was first recognized in 2004.
Biopsy
Liver biopsies are used to determine the degree of liver damage present; however, there are risks from the procedure. The typical changes seen are lymphocytes within the parenchyma, lymphoid follicles in portal triad, and changes to the bile ducts. There are a number of blood tests available that try to determine the degree of hepatic fibrosis and alleviate the need for biopsy.
Screening
It is believed that only 5–50% of those infected in the United States and Canada are aware of their status.
Testing is recommended for those at high risk, which includes injection
drug users, those who have received blood transfusions before 1992, those who have been in jail, those on long term hemodialysis, and those with tattoos. Screening is also recommended in those with elevated liver enzymes, as this is frequently the only sign of chronic hepatitis. Routine screening is not currently recommended in the United States. In 2012, the U.S. Centers for Disease Control and Prevention (CDC) added a recommendation for a single screening test for those born between 1945 and 1965. In Canada one time screening is recommended for those born between 1945 and 1975.
Prevention
As of 2016, no approved vaccine protects against contracting hepatitis C. A combination of harm reduction strategies, such as the provision of new needles and syringes and treatment of substance use, decreases the risk of hepatitis C in intravenous drug users by about 75%. The screening of blood donors is important at a national level, as is adhering to universal precautions within healthcare facilities. In countries where there is an insufficient supply of sterile syringes, medications should be given orally rather than via injection (when possible).
Treatment
HCV induces chronic infection in 80% of infected persons. Approximately 95% of these clear with treatment. In rare cases, infection can clear without treatment. Those with chronic hepatitis C are advised to avoid alcohol and medications toxic to the liver. They should also be vaccinated against hepatitis A and hepatitis B due to the increased risk if also infected. Use of acetaminophen is generally considered safe at reduced doses. Nonsteroidal anti-inflammatory drugs (NSAIDs) are not recommended in those with advanced liver disease due to an increased risk of bleeding. Ultrasound surveillance for hepatocellular carcinoma is recommended in those with accompanying cirrhosis. Coffee consumption has been associated with a slower rate of liver scarring in those infected with HCV.
Medications
Treatment with antiviral medication is recommended in all people with proven chronic hepatitis C who are not at high risk of dying from other causes.
People with the highest complication risk should be treated first, with
the risk of complications based on the degree of liver scarring.
The initial recommended treatment depends on the type of hepatitis C
virus, if the person has received previous hepatitis C treatment, and
whether or not a person has cirrhosis. Direct-acting antivirals (DAAs) may reduce the number of the infected people.
No prior treatment
- HCV genotype 1a (no cirrhosis): 8 weeks of glecaprevir/pibrentasvir or ledipasvir/sofosbuvir (the latter for people who do not have HIV/AIDS, are not African-American, and have less than 6 million HCV viral copies per milliliter of blood) or 12 weeks of elbasvir/grazoprevir, ledipasvir/sofosbuvir, or sofosbuvir/velpatasvir. Sofosbuvir with either daclatasvir or simeprevir may also be used.
- HCV genotype 1a (with compensated cirrhosis): 12 weeks of elbasvir/grazoprevir, glecaprevir/pibrentasvir, ledipasvir/sofosbuvir, or sofosbuvir/velpatasvir. An alternative treatment regimen of elbasvir/grazoprevir with weight-based ribavirin for 16 weeks can be used if the HCV is found to have antiviral resistance mutations against NS5A protease inhibitors.
- HCV genotype 1b (no cirrhosis): 8 weeks of glecaprevir/pibrentasvir or ledipasvir/sofosbuvir (with the aforementioned limitations for the latter as above) or 12 weeks of elbasvir/grazoprevir, ledipasvir/sofosbuvir, or sofosbuvir/velpatasvir. Alternative regimens include 12 weeks of ombitasvir/paritaprevir/ritonavir with dasabuvir or 12 weeks of sofosbuvir with either daclatasvir or simeprevir.
- HCV genotype 1b (with compensated cirrhosis): 12 weeks of elbasvir/grazoprevir, glecaprevir/pibrentasvir, ledipasvir/sofosbuvir, or sofosbuvir/velpatasvir. A 12-week course of paritaprevir/ritonavir/ombitasvir with dasabuvir may also be used.
- HCV genotype 2 (no cirrhosis): 8 weeks of glecaprevir/pibrentasvir or 12 weeks of sofosbuvir/velpatasvir. Alternatively, 12 weeks of sofosbuvir/daclatasvir can be used.
- HCV genotype 2 (with compensated cirrhosis): 12 weeks of sofosbuvir/velpatasvir or glecaprevir/pibrentasvir. An alternative regimen of sofosbuvir/daclatasvir can be used for 16–24 weeks.
- HCV genotype 3 (no cirrhosis): 8 weeks of glecaprevir/pibrentasvir or 12 weeks of sofosbuvir/velpatasvir or sofosbuvir and daclatasvir.
- HCV genotype 3 (with compensated cirrhosis): 12 weeks of glecaprevir/pibrentasvir, sofosbuvir/velpatasvir, or if certain antiviral mutations are present, 12 weeks of sofosbuvir/velpatasvir/voxilaprevir (when certain antiviral mutations are present), or 24 weeks of sofosbuvir and daclatasvir.
- HCV genotype 4 (no cirrhosis): 8 weeks of glecaprevir/pibrentasvir or 12 weeks of sofosbuvir/velpatasvir, elbasvir/grazoprevir, or ledipasvir/sofosbuvir. A 12-week regimen of ombitasvir/paritaprevir/ritonavir is also acceptable in combination with weight-based ribavirin.
- HCV genotype 4 (with compensated cirrhosis): A 12-week regimen of sofosbuvir/velpatasvir, glecaprevir/pibrentasavir, elbasvir/grazoprevir, or ledipasvir/sofosbuvir is recommended. A 12-week course of ombitasvir/paritaprevir/ritonavir with weight-based ribavirin is an acceptable alternative.
- HCV genotype 5 or 6 (with or without compensated cirrhosis): If no cirrhosis is present, then 8 weeks of glecaprevir/pibrentasvir is recommended. If cirrhosis is present, then a 12-week course of glecaprevir/pibrentasvir, sofosbuvir/velpatasvir, or ledipasvir/sofosbuvir is warranted.
Chronic infection can be cured about 95% of the time with recommended treatment in 2017. Getting access to these treatments however can be expensive.
The combination of sofosbuvir, velpatasvir, and voxilaprevir may be
used in those who have previously been treated with sofosbuvir or other
drugs that inhibit NS5A and were not cured.
Prior to 2011, treatments consisted of a combination of pegylated interferon alpha and ribavirin for a period of 24 or 48 weeks, depending on HCV genotype. This produces cure rates of between 70 and 80% for genotype 2 and 3, respectively, and 45 to 70% for genotypes 1 and 4. Adverse effects with these treatments were common, with half of people getting flu-like symptoms and a third experiencing emotional problems. Treatment during the first six months is more effective than once hepatitis C has become chronic. In those with chronic hepatitis B, treatment for hepatitis C results in reactivation of hepatitis B in about 25%.
Surgery
Cirrhosis due to hepatitis C is a common reason for liver transplantation though the virus usually (80–90% of cases) recurs afterwards. Infection of the graft leads to 10–30% of people developing cirrhosis within five years. Treatment with pegylated interferon and ribavirin post-transplant decreases the risk of recurrence to 70%. A 2013 review found unclear evidence regarding if antiviral medication was useful if the graft became reinfetcted.
Alternative medicine
Several alternative therapies are claimed by their proponents to be helpful for hepatitis C including milk thistle, ginseng, and colloidal silver. However, no alternative therapy has been shown to improve outcomes in hepatitis C, and no evidence exists that alternative therapies have any effect on the virus at all.
Prognosis
Disability-adjusted life year for hepatitis C in 2004 per 100,000 inhabitants
|
no data
<10 span="">
10–15
15–20
20–25
25–30
30–35
|
35–40
40–45
45–50
50–75
75–100
>100
|
The responses to treatment is measured by sustained viral response (SVR), defined as the absence of detectable RNA of the hepatitis C virus in blood serum for at least 24 weeks after discontinuing the treatment,
and rapid virological response (RVR) defined as undetectable levels
achieved within four weeks of treatment. Successful treatment decreases
the future risk of hepatocellular carcinoma by 75%.
Prior to 2012 sustained response occurs in about 40–50% in people with HCV genotype 1 given 48 weeks of treatment. A sustained response is seen in 70–80% of people with HCV genotypes 2 and 3 with 24 weeks of treatment.
A sustained response occurs about 65% in those with genotype 4 after 48
weeks of treatment. The evidence for treatment in genotype 6 disease is
sparse and what evidence there is supports 48 weeks of treatment at the
same doses used for genotype 1 disease.
Epidemiology
Prevalence of hepatitis C worldwide in 1999
It is estimated that 143 million people (2%) of people globally are living with chronic hepatitis C. About 3–4 million people are infected per year, and more than 350,000 people die yearly from hepatitis C-related diseases.
During 2010 it is estimated that 16,000 people died from acute
infections while 196,000 deaths occurred from liver cancer secondary to
the infection.
Rates have increased substantially in the 20th century due to a
combination of intravenous drug abuse and reused but poorly sterilized
medical equipment.
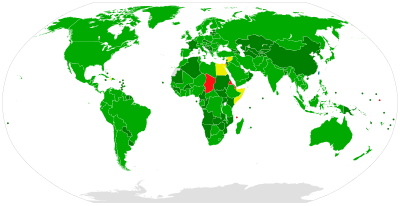
Rates are high (>3.5% population infected) in Central and East
Asia, North Africa and the Middle East, they are intermediate
(1.5%-3.5%) in South and Southeast Asia, sub-Saharan Africa, Andean,
Central and Southern Latin America, Caribbean, Oceania, Australasia and
Central, Eastern and Western Europe; and they are low (<1 .5="" america.="" america="" and="" asia-pacific="" in="" latin="" north="" p="" tropical="">
Among those chronically infected, the risk of cirrhosis
after 20 years varies between studies but has been estimated at ~10–15%
for men and ~1–5% for women. The reason for this difference is not
known. Once cirrhosis is established, the rate of developing hepatocellular carcinoma is ~1–4% per year.
Rates of new infections have decreased in the Western world since the
1990s due to improved screening of blood before transfusion.
In the United States, about 2% of people have chronic hepatitis C. In 2014, an estimated 30,500 new acute hepatitis C cases occurred (0.7 per 100,000 population), an increase from 2010–2012. The number of deaths from hepatitis C has increased to 15,800 in 2008 having overtaken HIV/AIDS as a cause of death in the USA in 2007. In 2014 it was the single greatest cause of infectious death in the United States. This mortality rate is expected to increase, as those infected by transfusion before HCV testing become apparent. In Europe the percentage of people with chronic infections has been estimated to be between 0.13 and 3.26%.
In England about 160,000 people are chronically infected. Between 2006 and 2011 28,000, about 3%, received treatment.
About half of people using a needle exchange in London in 2017/8 tested
positive for hepatitis C of which half were unaware of they had it. As part of a bid to eradicate hepatitis C by 2025 NHS England conducted a large procurement exercise in 2019. Merck Sharp & Dohme, Gilead Sciences, and Abbvie were awarded contracts, which, together, are worth up to £1 billion over five years.
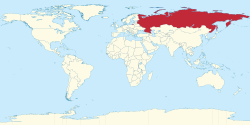
The total number of people with this infection is higher in some countries in Africa and Asia. Countries with particularly high rates of infection include Egypt (22%), Pakistan (4.8%) and China (3.2%). It is believed that the high prevalence in Egypt is linked to a now-discontinued mass-treatment campaign for schistosomiasis, using improperly sterilized glass syringes.
History
In the mid-1970s, Harvey J. Alter, Chief of the Infectious Disease Section in the Department of Transfusion Medicine at the National Institutes of Health, and his research team demonstrated how most post-transfusion hepatitis cases were not due to hepatitis A or B viruses. Despite this discovery, international research efforts to identify the virus, initially called non-A, non-B hepatitis (NANBH), failed for the next decade. In 1987, Michael Houghton, Qui-Lim Choo, and George Kuo at Chiron Corporation, collaborating with Daniel W. Bradley at the Centers for Disease Control and Prevention, used a novel molecular cloning approach to identify the unknown organism and develop a diagnostic test.
In 1988, Alter confirmed the virus by verifying its presence in a panel
of NANBH specimens. In April 1989, the discovery of HCV was published
in two articles in the journal Science. The discovery led to significant improvements in diagnosis and improved antiviral treatment. In 2000, Drs. Alter and Houghton were honored with the Lasker Award
for Clinical Medical Research for "pioneering work leading to the
discovery of the virus that causes hepatitis C and the development of
screening methods that reduced the risk of blood transfusion-associated
hepatitis in the U.S. from 30% in 1970 to virtually zero in 2000."
Chiron filed for several patents on the virus and its diagnosis.
A competing patent application by the CDC was dropped in 1990 after
Chiron paid $1.9 million to the CDC and $337,500 to Bradley. In 1994,
Bradley sued Chiron, seeking to invalidate the patent, have himself
included as a coinventor, and receive damages and royalty income. He
dropped the suit in 1998 after losing before an appeals court.
Society and culture
World Hepatitis Day, held on July 28, is coordinated by the World Hepatitis Alliance.
The economic costs of hepatitis C are significant both to the
individual and to society. In the United States the average lifetime
cost of the disease was estimated at 33,407 USD in 2003 with the cost of a liver transplant as of 2011 costing approximately 200,000 USD. In Canada the cost of a course of antiviral treatment is as high as 30,000 CAD in 2003, while the United States costs are between 9,200 and 17,600 in 1998 USD.
In many areas of the world, people are unable to afford treatment with
antivirals as they either lack insurance coverage or the insurance they
have will not pay for antivirals. In the English National Health Service treatment rates for hepatitis C are higher among wealthier groups per 2010–2012 data. Spanish anaesthetist Juan Maeso infected 275 patients between 1988 and 1997 as he used the same needles to give both himself and the patients opioids. For this he was jailed.
Special populations
Children and pregnancy
Compared with adults, infection in children is much less well
understood. Worldwide the prevalence of hepatitis C virus infection in
pregnant women and children has been estimated to 1–8% and 0.05–5%
respectively. The vertical transmission
rate has been estimated to be 3–5% and there is a high rate of
spontaneous clearance (25–50%) in the children. Higher rates have been
reported for both vertical transmission (18%, 6–36% and 41%). and prevalence in children (15%).
In developed countries transmission around the time of birth is
now the leading cause of HCV infection. In the absence of virus in the
mother's blood transmission seems to be rare.
Factors associated with an increased rate of infection include membrane
rupture of longer than 6 hours before delivery and procedures exposing
the infant to maternal blood.
Cesarean sections are not recommended. Breastfeeding is considered safe
if the nipples are not damaged. Infection around the time of birth in
one child does not increase the risk in a subsequent pregnancy. All
genotypes appear to have the same risk of transmission.
HCV infection is frequently found in children who have previously
been presumed to have non-A, non-B hepatitis and cryptogenic liver
disease. The presentation in childhood may be asymptomatic or with elevated liver function tests. While infection is commonly asymptomatic both cirrhosis with liver failure and hepatocellular carcinoma may occur in childhood.
Immunosuppressed
The rate of hepatitis C in immunosuppressed people is higher. This is particularly true in those with human immunodeficiency virus infection, recipients of organ transplants, and those with hypogammaglobulinemia.
Infection in these people is associated with an unusually rapid
progression to cirrhosis. People with stable HIV who never received
medication for HCV, may be treated with a combination of peginterferon plus ribavirin with caution to the possible side effects.
Research
As of 2011, there are about one hundred medications in development for hepatitis C. These include vaccines to treat hepatitis, immunomodulators, and cyclophilin inhibitors, among others. These potential new treatments have come about due to a better understanding of the hepatitis C virus. There are a number of vaccines under development and some have shown encouraging results.
The combination of sofosbuvir and velpatasvir in one trial (reported in 2015) resulted in cure rates of 99%.
More studies are needed to investigate the role of the preventative
antiviral medication against HCV recurrence after transplantation.
Animal models
One barrier to finding treatments for hepatitis C is the lack of a
suitable animal model. Despite moderate success, current research
highlights the need for pre-clinical testing in mammalian systems such
as mouse, particularly for the development of vaccines in poorer communities. Currently, chimpanzees
remain the available living system to study, yet their use has ethical
concerns and regulatory restrictions. While scientists have made use of
human cell culture systems such as hepatocytes, questions have been
raised about their accuracy in reflecting the body's response to
infection.
One aspect of hepatitis research is to reproduce infections in
mammalian models. A strategy is to introduce liver tissues from humans
into mice, a technique known as xenotransplantation. This is done by
generating chimeric mice, and exposing the mice HCV infection. This
engineering process is known to create humanized mice, and provide
opportunities to study hepatitis C within the 3D architectural design of
the liver and evaluating antiviral compounds. Alternatively, generating inbred mice with susceptibility to HCV would simplify the process of studying mouse models.