| ||||||
| Identifiers | ||||||
|---|---|---|---|---|---|---|
| Aliases | MAPT, DDPAC, FTDP-17, MAPTL, MSTD, MTBT1, MTBT2, PPND, PPP1R103, TAU, microtubule associated protein tau, Tau proteins | |||||
| External IDs | OMIM: 157140 MGI: 97180 HomoloGene: 74962 GeneCards: MAPT | |||||
| |||||||||||||||||||||||||
| |||||||||||||||
| |||||||
| Orthologs | |||||||
|---|---|---|---|---|---|---|---|
| Species | Human | Mouse | |||||
| Entrez | |||||||
| Ensembl | |||||||
| UniProt | |||||||
| RefSeq (mRNA) | |||||||
|
| ||
| RefSeq (protein) |
|---|
|
| ||
| Location (UCSC) | Chr 17: 45.89 – 46.03 Mb | Chr 11: 104.23 – 104.33 Mb |
|---|
Tau proteins (or τ proteins, after the Greek letter with that name) are proteins that stabilize microtubules. They are abundant in neurons of the central nervous system and are less common elsewhere, but are also expressed at very low levels in CNS astrocytes and oligodendrocytes. Pathologies and dementias of the nervous system such as Alzheimer's disease and Parkinson's disease are associated with tau proteins that have become defective and no longer stabilize microtubules properly.
The tau proteins are the product of alternative splicing from a single gene that in humans is designated MAPT (microtubule-associated protein tau) and is located on chromosome 17.
The tau proteins were identified in 1975 as heat-stable proteins essential for microtubule assembly and since then, they have been characterized as intrinsically disordered proteins.
Neurons were grown in tissue culture and stained with antibody to MAP2 protein in green and MAP tau in red using the immunofluorescence
technique. MAP2 is found only in dendrites and perikarya, while tau is
found not only in the dendrites and perikarya but also in axons. As a
result, axons appear red while the dendrites and perikarya appear
yellow, due to superimposition of the red and green signals. DNA is
shown in blue using the DAPI stain which highlights the nuclei.
Function
Tau protein is a highly soluble microtubule-associated protein
tau (MAPT). In humans, these proteins are found mostly in neurons
compared to non-neuronal cells. One of tau's main functions is to
modulate the stability of axonal microtubules. Other nervous system MAPs
may perform similar functions, as suggested by tau knock out mice that
did not show abnormalities in brain development - possibly because of
compensation in tau deficiency by other MAPs. Tau is not present in dendrites and is active primarily in the distal portions of axons where it provides microtubule stabilization but also flexibility as needed. This contrasts with MAP6 (STOP) proteins in the proximal portions of axons, which, in essence, lock down the microtubules and MAP2
that stabilizes microtubules in dendrites. In addition to their
microtubule stabilizing functions, MAPTs have also been found to recruit
signaling proteins and regulation of microtubule-mediated transport.
Tau proteins interact with tubulin to stabilize microtubules and promote tubulin assembly into microtubules. Tau has two ways of controlling microtubule stability: isoforms and phosphorylation.
Tau regulates cytoskeletal stability and translation (negatively) in Drosophila as well, but also facilitates habituation (a form of non-associative learning) and negatively regulates long-term memory, two higher and more integrated neural functions.
Genetics
In humans, the MAPT gene for encoding tau protein is located on chromosome 17q21, containing 16 exons. The major tau protein in the human brain is encoded by 11 exons. Exons 2, 3 and 10 are alternatively spliced that lead to formation of six tau isoforms. In human brain, tau proteins constitute a family of six isoforms with a range of 352-441 amino acids. Tau isoforms are different in either zero, one, or two inserts of 29 amino acids at the N-terminal part (exon 2 and 3), and three or four repeat-regions at the C-terminal part (exon 10). Thus, the longest isoform in the CNS
has four repeats (R1, R2, R3 and R4) and two inserts (441 amino acids
total), while the shortest isoform has three repeats (R1, R3 and R4) and
no insert (352 amino acids total).
The MAPT gene has two haplogroups,
H1 and H2, in which the gene appears in inverted orientations.
Haplogroup H2 is common only in Europe and in people with European
ancestry. Haplogroup H1 appears to be associated with increased
probability of certain dementias, such as Alzheimer's disease. The
presence of both haplogroups in Europe means that recombination between
inverted haplotypes can result in the lack of one of the functioning
copies of the gene, resulting in congenital defects.
Structure
Six tau isoforms exist in human brain tissue, and they are distinguished by their number of binding domains.
Three isoforms have three binding domains and the other three have
four binding domains. The binding domains are located in the carboxy-terminus
of the protein and are positively charged (allowing it to bind to the
negatively charged microtubule). The isoforms with four binding domains
are better at stabilizing microtubules than those with three binding
domains. The isoforms are a result of alternative splicing in exons 2, 3, and 10 of the tau gene. Tau is a phosphoprotein
with 79 potential Serine (Ser) and Threonine (Thr) phosphorylation
sites on the longest tau isoform. Phosphorylation has been reported on
approximately 30 of these sites in normal tau proteins.
Phosphorylation of tau is regulated by a host of kinases, including PKN, a serine/threonine kinase. When PKN is activated, it phosphorylates tau, resulting in disruption of microtubule organization.
Phosphorylation of tau is also developmentally regulated. For example,
fetal tau is more highly phosphorylated in the embryonic CNS than adult
tau. The degree of phosphorylation in all six isoforms decreases with age due to the activation of phosphatases.
Like kinases, phosphatases too play a role in regulating the
phosphorylation of tau. For example, PP2A and PP2B are both present in
human brain tissue and have the ability to dephosphorylate Ser396. The binding of these phosphatases to tau affects tau's association with MTs.
Mechanism
The accumulation of hyperphosphorylated tau in neurons leads to the neurofibrillary degeneration.
The actual mechanism of how tau propagates from one cell to another is
not well identified. Also, other mechanisms, including tau release and
toxicity, are unclear. As tau aggregates, it replaces tubulin, which in
turn enhances fibrilization of tau.
Several propagation methods have been proposed which occur by synaptic
contact such as synaptic cell adhesion proteins and neuronal activity
and other synaptic and non-synaptic mechanisms.
The mechanism of tau aggregation is still not completely elucidated,
but several factors favor this process, including tau phosphorylation
and zinc ions.
Release
Tau
involves in uptake and release process, which is known as seeding.
Uptake of tau protein mechanism requires the presence of heparan sulfate
proteoglycans at the cell surface, which happen by macropinocytosis.
On the other hand, tau release depends on neuronal activity. Many
factors influence tau release, for example, type of isoforms or MAPT mutations that change the extracellular level of tau.
According to Asai and his colleagues, spreading of tau protein occurs
from entorhinal cortex to the hippocampal region in the early stages of
the disease. They also suggested that microglia were also involved in
the transport process and their actual role is still unknown.
Toxicity
Tau
causes toxic effects through its accumulation inside cells. Many enzymes
are involved in toxicity mechanism such as PAR-1 kinase. This enzyme
stimulates phosphorylation of serine 262 and 356, which in turn leads to
activate other kinases (GSK-3 and Cdk5) that cause disease-associated
phosphoepitopes. The degree of toxicity is affected by different factors, such as the degree of microtubule binding. Toxicity could also happen by neurofibrillary tangles (NFTs), which leads to cell death and cognitive decline.
Clinical significance
Hyperphosphorylation of the tau protein (tau inclusions, pTau) can result in the self-assembly of tangles of paired helical filaments and straight filaments, which are involved in the pathogenesis of Alzheimer's disease, frontotemporal dementia, and other tauopathies.
All of the six tau isoforms are present in an often
hyperphosphorylated state in paired helical filaments from Alzheimer's
disease brain. In other neurodegenerative diseases, the deposition of aggregates enriched in certain tau isoforms has been reported. When misfolded,
this otherwise very soluble protein can form extremely insoluble
aggregates that contribute to a number of neurodegenerative diseases.
Tau protein has a direct effect on the breakdown of a living cell
caused by tangles that form and block nerve synapses. Tangles are
clumps of tau protein that stick together and block essential nutrients
that need to be distributed to cells in the brain, causing the cells to
die.
Gender-specific tau gene expression across different regions of
the human brain has recently been implicated in gender differences in
the manifestations and risk for tauopathies.
Some aspects of how the disease functions also suggest that it has some similarities to prion proteins.
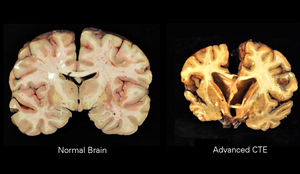
Traumatic brain injury
Repetitive mild traumatic brain injury (TBI) is now recognized as a central component of brain injury in contact sports, especially American football, and the concussive force of military blasts. It can lead to chronic traumatic encephalopathy (CTE) that is characterized by fibrillar tangles of hyperphosphorylated tau.
High levels of tau protein in fluid bathing the brain are linked to poor recovery after head trauma.
Tau hypothesis of Alzheimer's disease
The
tau hypothesis states that excessive or abnormal phosphorylation of tau
results in the transformation of normal adult tau into paired helical
filament (PHF) tau and neurofibrillary tangles (NFTs).
The stage of the disease determines NFTs' phosphorylation. In AD, at
least 19 amino acids are phosphorylated, such as pre-NFT phosphorylation
occurs at serine 119, 202, and 409. While intra-NFT phosphorylation
happens at serine 396 and threonine 231. Tau protein is a highly soluble microtubule-associated protein tau (MAPT).
Through its isoforms and phosphorylation, tau protein interacts with
tubulin to stabilize microtubule assembly. All of the six tau isoforms
are present in an often hyperphosphorylated state in paired helical
filaments (PHFs) from AD.
Tau mutations have many consequences such as changing the expression level of tau isoforms or lead to MTs dysfunction.
Mutations that alter function and isoform expression of tau lead to
hyperphosphorylation. The process of tau aggregation in the absence of
mutations is not known, but might result from increased phosphorylation,
protease
action, or exposure to polyanions, such as glycosaminoglycans.
Hyperphosphorylated tau disassembles microtubules and sequesters normal
tau, MAPT 1 (microtubule associated protein tau 1), MAPT
2, and ubiquitin into tangles of PHFs. This insoluble structure damages
cytoplasmic functions and interferes with axonal transport, which can
lead to cell death. Hyperphosphorylated
forms of tau protein are the main component of PHFs of NFTs in the
brain of AD patients. It has been well demonstrated that regions of tau
six-residue segments, namely PHF6 (VQIVYK) and PHF6 (VQIINK), can form
tau PHF aggregation in AD. Apart from the PHF6, some other residue sites
like Ser285, Ser289, Ser293, Ser305, and Tyr310, located near the
C-terminal of the PHF6 sequences, play key roles in the phosphorylation
of tau.
A68 Protein
The A68 protein is a hyperphosphorylated Tau protein differing in its sensitivity and its Kinase as well as Alkaline phosphatase and is along with beta-amyloid a component of pathologic lesions in Alzheimer disease, and is found in the brains of individuals with Alzheimer's disease.
Interactions
Tau protein has been shown to interact with proto-oncogene tyrosine-protein kinase:
- Alpha-synuclein,
- FYN,
- S100B, and
- YWHAZ.