| Chronic traumatic encephalopathy | |
|---|---|
| Other names | Traumatic encephalopathy syndrome, dementia pugilistica, punch drunk syndrome |
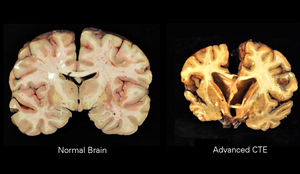 | |
| A normal brain (left) and one with CTE (right) | |
| Specialty | Neurology, psychiatry, sports medicine |
| Symptoms | Behavioral problems, mood problems, problems with thinking |
| Complications | Dementia, aggression, depression, suicidal thoughts |
| Usual onset | Years after initial injuries |
| Causes | Repeated head injuries |
| Risk factors | Contact sports, military, domestic abuse, repeated banging of the head |
| Diagnostic method | Autopsy |
| Differential diagnosis | Alzheimer's disease, Parkinson's disease |
| Treatment | Supportive care |
| Frequency | Uncertain |
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease caused by repeated head injuries. Symptoms may include behavioral problems, mood problems, and problems with thinking. Symptoms typically do not begin until years after the injuries. CTE often gets worse over time and can result in dementia. It is unclear if the risk of suicide is altered.
Most documented cases have occurred in athletes involved in contact sports such as boxing, American football, professional wrestling, ice hockey, rugby and soccer. Other risk factors include being in the military, prior domestic violence, and repeated banging of the head. The exact amount of trauma required for the condition to occur is unknown. Definitive diagnosis can only occur at autopsy. Chronic traumatic encephalopathy is a form of tauopathy.
There is no specific treatment. Rates of disease have been found to be about 30% among those with a history of multiple head injuries. Population rates, however, are unclear. Research in brain damage as a result of repeated head injuries began in the 1920s, at which time the condition was known as dementia pugilistica or "punch drunk syndrome". Changing the rules in some sports has been discussed as a means of prevention.
Signs and symptoms
Symptoms
of CTE, which occur in four stages, generally appear eight to ten years
after an individual experiences repetitive mild traumatic brain
injuries.
First-stage symptoms include attention deficit hyperactivity disorder as well as confusion, disorientation, dizziness, and headaches. Second-stage symptoms include memory loss, social instability, impulsive behavior, and poor judgment. Third and fourth stages include progressive dementia, movement disorders, hypomimia, speech impediments, sensory processing disorder, tremors, vertigo, deafness, depression and suicidality.
Additional symptoms include dysarthria, dysphagia, cognitive disorders such as amnesia, and ocular abnormalities, such as ptosis.
The condition manifests as dementia,
or declining mental ability, problems with memory, dizzy spells or lack
of balance to the point of not being able to walk under one's own power
for a short time and/or Parkinsonism, or tremors and lack of coordination. It can also cause speech problems and an unsteady gait. Patients with CTE may be prone to inappropriate or explosive behavior and may display pathological jealousy or paranoia.
Causes
Most documented cases have occurred in athletes with mild repetitive
brain trauma (RBT) over an extended period of time. Specifically contact sports such as boxing, American football, wrestling, ice hockey, rugby, and football (soccer). In soccer whether this is just associated with prolific headers or other injuries is unclear as of 2017. Other potential risk factors include military personnel (repeated exposure to concussions charges or large caliber ordnance), domestic violence, and repeated banging of the head. The exact amount of trauma required for the condition to occur is unknown.
Pathology
The neuropathological appearance of CTE is distinguished from other tauopathies, such as Alzheimer's disease.
The four clinical stages of observable CTE disability have been
correlated with tau pathology in brain tissue, ranging in severity from
focal perivascular epicenters of neurofibrillary tangles in the frontal
neocortex to severe tauopathy affecting widespread brain regions.
The primary physical manifestations of CTE include a reduction in
brain weight, associated with atrophy of the frontal and temporal
cortices and medial temporal lobe. The lateral ventricles and the third ventricle are often enlarged, with rare instances of dilation of the fourth ventricle.[10] Other physical manifestations of CTE include anterior cavum septi pellucidi and posterior fenestrations, pallor of the substantia nigra and locus ceruleus, and atrophy of the olfactory bulbs, thalamus, mammillary bodies, brainstem and cerebellum. As CTE progresses, there may be marked atrophy of the hippocampus, entorhinal cortex, and amygdala.
On a microscopic scale, the pathology includes neuronal loss, tau deposition, TAR DNA-binding Protein 43 (TDP 43) deposition, white matter changes, and other abnormalities. The tau deposition occurs as dense neurofibrillary tangles (NFT), neurites, and glial tangles, which are made up of astrocytes and other glial cells Beta-amyloid deposition is a relatively uncommon feature of CTE.
A small group of individuals with CTE have chronic traumatic
encephalomyopathy (CTEM), which is characterized by symptoms of
motor-neuron disease and which mimics amyotrophic lateral sclerosis (ALS). Progressive muscle weakness and balance and gait problems (problems with walking) seem to be early signs of CTEM.
Exosome vesicles created by the brain are potential biomarkers of TBI, including CTE. A subtype of CTE is dementia pugilistica or boxer's dementia (from Latin pugilator - boxer) as it was initially found in those with a history of boxing, also called "punch-drunk syndrome".
Loss of neurons, scarring of brain tissue, collection of proteinaceous, senile plaques, hydrocephalus, attenuation of the corpus callosum, diffuse axonal injury, neurofibrillary tangles, and damage to the cerebellum are implicated in the syndrome. The condition may be etiologically related to Alzheimer's disease.
Neurofibrillary tangles have been found in the brains of dementia
pugilistica patients, but not in the same distribution as is usually
found in people with Alzheimer's.
One group examined slices of brain from patients having had multiple
mild traumatic brain injuries and found changes in the cells' cytoskeletons, which they suggested might be due to damage to cerebral blood vessels.
Increased exposure to concussions and sub-concussive blows is
regarded as the most important risk factor, which can depend on the
total number of fights, number of knockout losses, the duration of
career, fight frequency, age of retirement, and boxing style.
Diagnosis
Diagnosis of CTE cannot be made in living individuals. A clear diagnosis is possible during an autopsy.
Though there are signs and symptoms some researchers associate with
CTE, there is no definitive test to prove the existence in a living
person. Signs are also very similar to that of other neurological
conditions such as Alzheimer's.
The lack of distinct biomarkers
is the reason CTE cannot typically be diagnosed while a person is
alive. Concussions are non-structural injuries and do not result in
brain bleeding, which is why most concussions cannot be seen on routine
neuroimaging tests such as CT or MRI.
Acute concussion symptoms (those that occur shortly after an injury)
should not be confused with CTE. Differentiating between prolonged
post-concussion syndrome (PCS, where symptoms begin shortly after a
concussion and last for weeks, months, and sometimes even years) and CTE
symptoms can be difficult. Research studies are currently examining
whether neuroimaging can detect subtle changes in axonal integrity and structural lesions that can occur in CTE. Recently, more progress in in-vivo diagnostic techniques for CTE has been made, using DTI, fMRI, MRI, and MRS imaging; however, more research needs to be done before any such techniques can be validated.
PET tracers that bind specifically to tau protein are desired to aid diagnosis of CTE in living individuals. One candidate is the tracer [18F]FDDNP, which is retained in the brain in individuals with a number of dementing disorders such as Alzheimer's disease, Down syndrome, progressive supranuclear palsy, corticobasal degeneration, familial frontotemporal dementia, and Creutzfeldt–Jakob disease.
In a small study of 5 retired NFL players with cognitive and mood
symptoms, the PET scans revealed accumulation of the tracer in their
brains. However, [18F]FDDNP binds to beta-amyloid
and other proteins as well. Moreover, the sites in the brain where the
tracer was retained were not consistent with the known neuropathology of
CTE. A more promising candidate is the tracer [18F]-T807, which binds only to tau. It is being tested in several clinical trials.
A putative biomarker for CTE is the presence in serum of
autoantibodies against the brain. The autoantibodies were detected in
football players who experienced a large number of head hits but no
concussions, suggesting that even sub-concussive episodes may be
damaging to the brain. The autoantibodies may enter the brain by means
of a disrupted blood-brain barrier, and attack neuronal cells which are normally protected from an immune onslaught.
Given the large numbers of neurons present in the brain (86 billion),
and considering the poor penetration of antibodies across a normal
blood-brain barrier, there is an extended period of time between the
initial events (head hits) and the development of any signs or symptoms.
Nevertheless, autoimmune changes in blood of players may consist the
earliest measurable event predicting CTE.
Imaging
Although
the diagnosis of CTE cannot be determined by imagining, the effects of
head trauma may be seen with the use of structural imaging. Imaging techniques include the use of magnetic resonance imaging, nuclear magnetic resonance spectroscopy, CT scan, single-photon emission computed tomography, Diffusion MRI, and Positron Emission Tomography (PET). One specific use of imaging is the use of a PET scan is to evaluate for tau deposition, most commonly conducted on retired NFL players
Prevention
Prevention of CTE in sport is not an idealistic goal because repetitive concussions increase the risk for this condition. Prevention techniques are also difficult because diagnosis of the condition can only be during a postmortem autopsy.
The initial onset of this condition can not yet be determined, and
therefore creating techniques for prevention pose a struggle.
Some common preventative methods have been the utilization of
helmets and mouth-guards, though neither have significant research to
support their use, they have shown prevention in direct head trauma.
Although there is no significant research to support the use of helmets
to reduce the risk of concussions, there is evidence to support that
helmet use lowers the impactive forces. Mouth guards have been shown to
decrease dental injuries, but again have not shown significant evidence
to reduce concussions.
A growing area of practice is the improved recognition and treatment
for concussions and other head trauma, because repeated impacts are
thought to increase the likelihood of CTE development, removal from
sport during these traumatic incidences is essential. Proper return to play protocol during brain injuries is also important to decrease the significance of future impacts.
Another factor that has been implemented and continues to be an
area of debate is to change the rules of many contact sports to make the
effectively safer.
Examples of these rules are the evolution of tackle technique rules in
American football, such as the banning of helmet-first tackles, and
addition of rules to protect defenseless players. Likewise, another
growing area of debate is the better implementation of current rules
that have previously been put in place to protect athletes.
Because of the concern that boxing may cause CTE, there is a movement among medical professionals to ban the sport. Medical professionals have called for such a ban since as early as the 1950s.
Management
No cure currently exists for CTE. Treatment is supportive as with other forms of dementia. Those with CTE-related symptoms may receive medication and non medication related treatments.
Epidemiology
Rates of disease have been found to be about 30% among those with a history of multiple head injuries. Population rates, however, are unclear.
Professional level athletes are the largest group with CTE, due to frequent concussions and sub-concussive impacts from play in contact sport. These contact-sports include American football, ice hockey, rugby, boxing, mixed martial arts, association football, wrestling, and war veterans. In association football, only prolific headers are known to have developed CTE.
Other individuals diagnosed with CTE were those involved in
military service, had a previous history of chronic seizures, were
domestically abused, or were involved in activities resulting in
repetitive head collisions.
History
CTE was originally studied in boxers in the 1920s as dementia pugilistica. DP was first described in 1928 by a forensic pathologist, Dr. Harrison Stanford Martland, who was the chief medical examiner of Essex County in Newark, New Jersey in a Journal of the American Medical Association article, in which he noted the tremors, slowed movement, confusion, and speech problems typical of the condition. The initial diagnosis of dementia pugilistica was derived from the Latin word for boxer pugil (akin to pugnus ‘fist’, pugnāre ‘to fight’).
Other terms for the condition have included chronic boxer's
encephalopathy, traumatic boxer's encephalopathy, boxer's dementia,
pugilistic dementia, chronic traumatic brain injury associated with
boxing (CTBI-B), and punch-drunk syndrome.
The seminal work on the disease came from British neurologist
Macdonald Critchley, who in 1949 wrote a paper titled "Punch-drunk
syndromes: the chronic traumatic encephalopathy of boxers."
CTE was first recognized as affecting individuals who took considerable
blows to the head, but was believed to be confined to boxers and not
other athletes. As evidence pertaining to the clinical and
neuropathological consequences of repeated mild head trauma grew, it
became clear that this pattern of neurodegeneration was not restricted
to boxers, and the term chronic traumatic encephalopathy became most
widely used. In the early 2000s, Nigerian-American neuropathologist Bennet Omalu worked on the case of American football player Mike Webster,
who died following unusual and unexplained behavior. In 2005 Omalu,
along with colleagues in the Department of Pathology at the University of Pittsburgh, published his findings in the journal Neurosurgery
in a paper which he titled "Chronic Traumatic Encephalopathy in a
National Football League Player." This was followed by a paper on a
second case in 2006 describing similar pathology.
In 2008, the Sports Legacy Institute joined with the Boston University School of Medicine (BUSM) to form the Center for the Study of Traumatic Encephalopathy (CSTE). Brain Injury Research Institute (BIRI) also studies the impact of concussions.
Research
In 2005 forensic pathologist Bennet Omalu,
along with colleagues in the Department of Pathology at the University
of Pittsburgh, published a paper, "Chronic Traumatic Encephalopathy in a
National Football League Player", in the journal Neurosurgery, based on analysis of the brain of deceased former NFL center Mike Webster.
This was then followed by a paper on a second case in 2006 describing
similar pathology, based on findings in the brain of former NFL player Terry Long.
In 2008, the CSTE at Boston University at the BU School of
Medicine started the CSTE brain bank at the Bedford VA Hospital to
analyze the effects of CTE and other neurodegenerative diseases on the
brain and spinal cord of athletes, military veterans, and civilians To date, the CSTE Brain Bank is the largest CTE tissue repository in the world.
On December 21, 2009, the National Football League Players Association announced that it would collaborate with the CSTE at the Boston University School of Medicine to support the Center's study of repetitive brain trauma in athletes. Additionally, in 2010 the National Football League gave the CSTE a $1 million gift with no strings attached. In 2008, twelve living athletes (active and retired), including hockey players Pat LaFontaine and Noah Welch as well as former NFL star Ted Johnson, committed to donate their brains to CSTE after their deaths. In 2009, NFL Pro Bowlers Matt Birk, Lofa Tatupu, and Sean Morey pledged to donate their brains to the CSTE.
In 2010, 20 more NFL players and former players pledged to join the
CSTE Brain Donation Registry, including Chicago Bears linebacker Hunter Hillenmeyer, Hall of Famer Mike Haynes, Pro Bowlers Zach Thomas, Kyle Turley, and Conrad Dobler, Super Bowl Champion Don Hasselbeck and former pro players Lew Carpenter, and Todd Hendricks. In 2010, Professional Wrestlers Mick Foley, Booker T and Matt Morgan also agreed to donate their brains upon their deaths. Also in 2010, MLS player Taylor Twellman,
who had to retire from the New England Revolution because of
post-concussion symptoms, agreed to donate his brain upon his death. As
of 2010, the CSTE Brain Donation Registry consists of over 250 current
and former athletes. In 2011, former North Queensland Cowboys player Shaun Valentine
became the first rugby league player to agree to donate his brain upon
his death, in response to recent concerns about the effects of
concussions on Rugby League players, who do not use helmets. Also in
2011, boxer Micky Ward, whose career inspired the film The Fighter, agreed to donate his brain upon his death.
In related research, the Center for the Study of Retired
Athletes, which is part of the Department of Exercise and Sport Science
at the University of North Carolina at Chapel Hill,
is conducting research funded by National Football League Charities to
"study former football players, a population with a high prevalence of
exposure to prior Mild Traumatic Brain Injury (MTBI) and sub-concussive
impacts, in order to investigate the association between increased
football exposure and recurrent MTBI and neurodegenerative disorders
such as cognitive impairment and Alzheimer's disease (AD)".
In February 2011, Dave Duerson committed suicide, leaving text messages to loved ones asking that his brain be donated to research for CTE. The family got in touch with representatives of the Boston University
center studying the condition, said Robert Stern, the co-director of
the research group. Stern said Duerson's gift was the first time of
which he was aware that such a request had been made by someone who had
committed suicide that was potentially linked to CTE.
Stern and his colleagues found high levels of the protein tau in
Duerson's brain. These elevated levels, which were abnormally clumped
and pooled along the brain sulci, are indicative of CTE.
In July 2010, NHL enforcer Bob Probert
died of heart failure. Before his death, he asked his wife to donate
his brain to CTE research because it was noticed that Probert
experienced a mental decline in his 40s. In March 2011, researchers at
Boston University concluded that Probert had CTE upon analysis of the
brain tissue he donated. He is the second NHL player from the program at
the Center for the Study of Traumatic Encephalopathy to be diagnosed
with CTE postmortem.
BUSM has also found indications of links between amyotrophic lateral sclerosis
(ALS) and CTE in athletes who have participated in contact sports.
Tissue for the study was donated by twelve athletes and their families
to the CSTE Brain Bank at the Bedford, Massachusetts VA Medical Center.
In 2013, President Barack Obama announced the creation of the Chronic Effects of Neurotrauma Consortium or CENC, a federally funded research project devised to address the long-term effects of mild traumatic brain injury in military service personnel (SM's) and Veterans. The CENC is a multi-center collaboration linking premiere basic
science, translational, and clinical neuroscience researchers from the
DoD, VA, academic universities, and private research institutes to
effectively address the scientific, diagnostic, and therapeutic
ramifications of mild TBI and its long-term effects. Nearly 20% of the more than 2.5 million U.S. Service Members (SMs) deployed since 2003 to Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) have sustained at least one traumatic brain injury (TBI), predominantly mild TBI (mTBI), and almost 8% of all OEF/OIF Veterans demonstrate persistent post-TBI symptoms more than six months post-injury.
Unlike those head injuries incurred in most sporting events, recent
military head injuries are most often the result of blast wave exposure. After a competitive application process, a consortium led by Virginia Commonwealth University was awarded funding. The project principal investigator for the CENC is David Cifu, Chairman and Herman J. Flax professor of the Department of Physical Medicine and Rehabilitation (PM&R) at Virginia Commonwealth University (VCU) in Richmond, Virginia, with co-principal investigators Ramon Diaz-Arrastia, Professor of Neurology, Uniformed Services University of the Health Sciences, and Rick L. Williams, statistician at RTI International.
