| Nephron | |
|---|---|

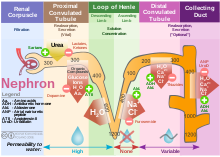
Diagram (left) of a long juxtamedullary nephron and (right) of a short cortical nephron.
The left nephron is labelled with six named nephron segments. Also
labelled is the collecting duct, mislabelled the "collection duct"; it
is not part of the nephron.
| |
| Details | |
| Precursor | Metanephric blastema (intermediate mesoderm) |
| System | Urinary system |
| Identifiers | |
| Latin | Nephroneum |
| MeSH | D009399 |
| FMA | 17640 |
The nephron is the microscopic structural and functional unit of the kidney. It is composed of a renal corpuscle and a renal tubule. The renal corpuscle consists of a tuft of capillaries called a glomerulus and an encompassing Bowman's capsule. The renal tubule extends from the capsule. The capsule and tubule are connected and are composed of epithelial cells with a lumen. A healthy adult has 0.8 to 1.5 million nephrons in each kidney. Blood is filtered as it passes through three layers: the endothelial cells of the capillary wall, its basement membrane, and between the foot processes of the podocytes of the lining of the capsule. The tubule has adjacent peritubular capillaries that run between the descending and ascending portions of the tubule. As the fluid from the capsule flows down into the tubule, it is processed by the epithelial cells lining the tubule: water is reabsorbed and substances are exchanged (some are added, others are removed); first with the interstitial fluid outside the tubules, and then into the plasma in the adjacent peritubular capillaries through the endothelial cells lining that capillary. This process regulates the volume of body fluid as well as levels of many body substances. At the end of the tubule, the remaining fluid—urine—exits: it is composed of water, metabolic waste, and toxins.
The interior of Bowman's capsule, called Bowman's space, collects the filtrate from the filtering capillaries of the glomerular tuft, which also contains mesangial cells supporting these capillaries. These components function as the filtration unit and make up the renal corpuscle. The filtering structure (glomerular filtration barrier) has three layers composed of endothelial cells, a basement membrane, and podocytes (foot processes). The tubule has five anatomically and functionally different parts: the proximal tubule, which has a convoluted section the proximal convoluted tubule followed by a straight section (proximal straight tubule); the loop of Henle, which has two parts, the descending loop of Henle ("descending loop") and the ascending loop of Henle ("ascending loop"); the distal convoluted tubule ("distal loop"); the connecting tubule, and the collecting ducts. Nephrons have two lengths with different urine concentrating capacities: long juxtamedullary nephrons and short cortical nephrons.
The four mechanisms used to create and process the filtrate (the result of which is to convert blood to urine) are filtration, reabsorption, secretion and excretion. Filtration occurs in the glomerulus and is largely passive: it is dependent on the intracapillary blood pressure. About one-fifth of the plasma is filtered as the blood passes through the glomerular capillaries; four-fifths continues into the peritubular capillaries. Normally the only components of the blood that are not filtered into Bowman's capsule are blood proteins, red blood cells, white blood cells and platelets. Over 150 liters of fluid enter the glomeruli of an adult every day: 99% of the water in that filtrate is reabsorbed. Reabsorption occurs in the renal tubules and is either passive, due to diffusion, or active, due to pumping against a concentration gradient. Secretion also occurs in the tubules and is active. Substances reabsorbed include: water, sodium chloride, glucose, amino acids, lactate, magnesium, calcium phosphate, uric acid, and bicarbonate. Substances secreted include urea, creatinine, potassium, hydrogen, and uric acid. Some of the hormones which signal the tubules to alter the reabsorption or secretion rate, and thereby maintain homeostasis, include (along with the substance affected) antidiuretic hormone (water), aldosterone (sodium, potassium), parathyroid hormone (calcium, phosphate), atrial natriuretic peptide (sodium) and brain natriuretic peptide (sodium). A countercurrent system in the renal medulla provides the mechanism for generating a hypertonic interstitium, which allows the recovery of solute-free water from within the nephron and returning it to the venous vasculature when appropriate.
Some diseases of the nephron predominantly affect either the glomeruli or the tubules. Glomerular diseases include diabetic nephropathy, glomerulonephritis and IgA nephropathy; renal tubular diseases include acute tubular necrosis and polycystic kidney disease.
Structure
Fig.1)
Schematic diagram of the nephron (yellow), relevant circulation
(red/blue), and the four methods of altering the filtrate.
The nephron is the functional unit of the kidney. Each nephron is composed of a renal corpuscle, the initial filtering component; and a renal tubule that processes and carries away the filtered fluid.
Renal corpuscle
Fig.2)
Schematic of the glomerular filtration barrier (GFB). A. The
endothelial cells of the glomerulus; 1. endothelial pore (fenestra).
B. Glomerular basement membrane: 1. lamina rara interna 2. lamina densa 3. lamina rara externa.
C. Podocytes: 1. enzymatic and structural proteins 2. filtration slit 3. diaphragm.
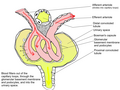
The renal corpuscle is the site of the filtration of blood plasma. The renal corpuscle consists of the glomerulus, and the glomerular capsule or Bowman's capsule.
The renal corpuscle has two poles – a vascular pole and a urinary pole.
The arterioles from the renal circulation
enter and leave the glomerulus at the vascular pole. The glomerular
filtrate leaves the Bowman's capsule at the renal tubule at the urinary
pole.
Glomerulus
The glomerulus is the network known as a tuft, of filtering capillaries located at the vascular pole of the renal corpuscle in Bowman's capsule. Each glomerulus receives its blood supply from an afferent arteriole of the renal circulation. The glomerular blood pressure provides the driving force for water and solutes to be filtered out of the blood plasma, and into the interior of Bowman's capsule, called Bowman's space.
Only about a fifth of the plasma is filtered in the glomerulus. The rest passes into an efferent arteriole.
The diameter of the efferent arteriole is smaller than that of the
afferent, and this difference increases the hydrostatic pressure in the
glomerulus.
Bowman's capsule
The Bowman's capsule,
also called the glomerular capsule, surrounds the glomerulus. It is
composed of a visceral inner layer formed by specialized cells called podocytes, and a parietal outer layer composed of simple squamous epithelium. Fluids from blood in the glomerulus are filtered through the visceral layer of podocytes, resulting in the glomerular filtrate.
The glomerular filtrate next moves to the renal tubule, where it is further processed to form urine. The different stages of this fluid are collectively known as the tubular fluid.
Renal tubule
The renal tubule is the portion of the nephron containing the tubular fluid filtered through the glomerulus. After passing through the renal tubule, the filtrate continues to the collecting duct system.
The components of the renal tubule are:
- Proximal convoluted tubule (lies in cortex and lined by simple cuboidal epithelium with brush borders which help to increase the area of absorption greatly.)
- Loop of Henle (hair-pin like, i.e. U-shaped, and lies in medulla)
- Descending limb of loop of Henle
- Ascending limb of loop of Henle
- The ascending limb of loop of Henle is divided into 2 segments: Lower end of ascending limb is very thin and is lined by simple squamous epithelium. The distal portion of ascending limb is thick and is lined by simple cuboidal epithelium.
- Thin ascending limb of loop of Henle
- Thick ascending limb of loop of Henle (enters cortex and becomes - distal convoluted tubule.)
- Distal convoluted tubule
- Connecting tubule
Blood from the efferent arteriole, containing everything that was not filtered out in the glomerulus, moves into the peritubular capillaries,
tiny blood vessels that surround the loop of Henle and the proximal and
distal tubules, where the tubular fluid flows. Substances then reabsorb
from the latter back to the blood stream.
The peritubular capillaries then recombine to form an efferent
venule, which combines with efferent venules from other nephrons into
the renal vein, and rejoins the main bloodstream.
Length difference
Cortical nephrons
(the majority of nephrons) start high in the cortex and have a short
loop of Henle which does not penetrate deeply into the medulla. Cortical
nephrons can be subdivided into superficial cortical nephrons and midcortical nephrons.
Juxtamedullary nephrons start low in the cortex near the medulla
and have a long loop of Henle which penetrates deeply into the renal
medulla: only they have their loop of Henle surrounded by the vasa recta.
These long loops of Henle and their associated vasa recta create a
hyperosmolar gradient that allows for the generation of a concentrated urine. Also the hairpin bend penetrates up to the inner zone of medulla.
Juxtamedullary nephrons are found only in birds and mammals, and have a specific location: medullary refers to the renal medulla, while juxta (Latin: near) refers to the relative position of the renal corpuscle of this nephron - near the medulla, but still in the cortex. In other words, a juxtamedullary nephron is a nephron whose renal corpuscle is near the medulla, and whose proximal convoluted tubule and its associated loop of Henle occur deeper in the medulla than the other type of nephron, the cortical nephron.
The juxtamedullary nephron comprises only 20–30% of the nephrons
in the human kidney. However, it is this type of nephron which is most
often depicted in illustrations of nephrons.
Functions
Fig.3) Secretion and reabsorption of various substances throughout the nephron.
The nephron uses four mechanisms to convert blood into urine:
filtration, reabsorption, secretion, and excretion of numerous
substances. The structure and function of the epithelial cells lining
the lumen change during the course of the nephron, and have segments
named by their location and which reflects their different functions.
Fig.4) Diagram outlining movement of ions in nephron, with the collecting ducts on the right.
Fig.5) Proximal tubule cell showing pumps involved in acid base balance, left is the lumen of tubule
Proximal convoluted tubule
The
proximal tubule as a part of the nephron can be divided into an initial
convoluted portion and a following straight (descending) portion.
Fluid in the filtrate entering the proximal convoluted tubule is
reabsorbed into the peritubular capillaries, including approximately
two-thirds of the filtered salt and water and all filtered organic solutes (primarily glucose and amino acids).
Loop of Henle
The loop of Henle
is a U-shaped tube that extends from the proximal tubule. It consists
of a descending limb and an ascending limb. It begins in the cortex,
receiving filtrate from the proximal convoluted tubule, extends into the
medulla as the descending limb, and then returns to the cortex as the
ascending limb to empty into the distal convoluted tubule. The primary
role of the loop of Henle is to concentrate the salt in the
interstitium, the tissue surrounding the loop.
Considerable differences aid in distinguishing the descending and ascending limbs of the loop of Henle. The descending limb
is permeable to water and noticeably less permeable to salt, and thus
only indirectly contributes to the concentration of the interstitium. As
the filtrate descends deeper into the hypertonic interstitium of the renal medulla, water flows freely out of the descending limb by osmosis
until the tonicity of the filtrate and interstitium equilibrate. The
hypertonicity of the medulla (and therefore concentration of urine) is
determined in part by the size of the loop of Henle.
Unlike the descending limb, the thin ascending limb is impermeable to water, a critical feature of the countercurrent exchange
mechanism employed by the loop. The ascending limb actively pumps
sodium out of the filtrate, generating the hypertonic interstitium that
drives countercurrent exchange. In passing through the ascending limb,
the filtrate grows hypotonic since it has lost much of its sodium content. This hypotonic filtrate is passed to the distal convoluted tubule in the renal cortex.
Distal convoluted tubule
The distal convoluted tubule has a different structure and function to that of the proximal convoluted tubule. Cells lining the tubule have numerous mitochondria to produce enough energy (ATP) for active transport to take place. Much of the ion transport taking place in the distal convoluted tubule is regulated by the endocrine system. In the presence of parathyroid hormone, the distal convoluted tubule reabsorbs more calcium and secretes more phosphate. When aldosterone is present, more sodium is reabsorbed and more potassium secreted. Atrial natriuretic peptide causes the distal convoluted tubule to secrete more sodium.
Connecting tubule
This is the final segment of the tubule before it enters the collecting duct system.
Collecting duct system
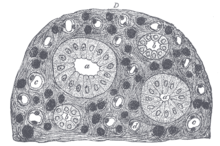
Fig.6)
Cross-sectional histologic preparation showing (b)small connecting
tubules with simple columnar epithelium and (a) large connecting tubules
with simple cuboidal epithelium.
Each distal convoluted tubule delivers its filtrate to a system of collecting ducts, the first segment of which is the connecting tubule.
The collecting duct system begins in the renal cortex and extends deep
into the medulla. As the urine travels down the collecting duct system,
it passes by the medullary interstitium which has a high sodium
concentration as a result of the loop of Henle's countercurrent multiplier system.
Because it has a different origin during the development of the urinary and reproductive organs
than the rest of the nephron, the collecting duct is sometimes not
considered a part of the nephron. Instead of originating from the
metanephrogenic blastema, the collecting duct originates from the ureteric bud.
Though the collecting duct is normally impermeable to water, it becomes permeable in the presence of antidiuretic hormone (ADH). ADH affects the function of aquaporins,
resulting in the reabsorption of water molecules as it passes through
the collecting duct. Aquaporins are membrane proteins that selectively
conduct water molecules while preventing the passage of ions and other
solutes. As much as three-quarters of the water from urine can be
reabsorbed as it leaves the collecting duct by osmosis. Thus the levels
of ADH determine whether urine will be concentrated or diluted. An
increase in ADH is an indication of dehydration, while water sufficiency results in a decrease in ADH allowing for diluted urine.
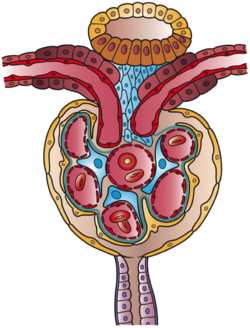
Fig.7)
Cross-sectional diagram of the juxtaglomerular apparatus and adjacent
structures: 1) top, yellow - distal convoluted tubule; 2) top, brown
-macula densa cuboidal cells surrounding arterioles; 3) small blue cells
- juxtaglomerular cells; 4) large blue cells - mesangial cells; 5) tan -
podocytes lining Bowman's capsule
adjacent to capillaries, and parietal layer of capsule, 6)center - five
glomerular capillaries, and the 6)bottom, purple - exiting tubule.
Structures (2), (3), and (4) constitute the juxtaglomerular apparatus.
Lower portions of the collecting organ are also permeable to urea, allowing some of it to enter the medulla of the kidney, thus maintaining its high concentration (which is very important for the nephron).
Urine leaves the medullary collecting ducts through the renal papillae, emptying into the renal calyces, the renal pelvis, and finally into the urinary bladder via the ureter.
Juxtaglomerular apparatus
The juxtaglomerular apparatus
(JGA) is a specialized region associated with the nephron, but separate
from it. It produces and secretes into the circulation the enzyme renin (angiotensinogenase), which cleaves angiotensinogen
and results in the ten amino acid substance angiotensin-1 (A-1). A-1
is then converted to angiotensin-2, a potent vasoconstrictor, by
removing two amino acids: this is accomplished by angiotensin converting
enzyme (ACE). This sequence of events is referred to as the renin–angiotensin system
(RAS) or renin-angiotensin-aldosterone system (RAAS). The JGA is
located between the thick ascending limb and the afferent arteriole. It
contains three components: the macula densa, juxtaglomerular cells, and extraglomerular mesangial cells.
Clinical significance
Diseases of the nephron predominantly affect either the glomeruli or the tubules. Glomerular diseases include diabetic nephropathy, glomerulonephritis and IgA nephropathy; renal tubular diseases include acute tubular necrosis, renal tubular acidosis, and polycystic kidney disease.