People maintaining social distance while queuing
to enter a supermarket in London during the 2020 COVID-19 pandemic. To
ensure that shoppers are able to maintain distance once in the store,
only a limited number are allowed inside at one time.
Social distancing reduces the rate of disease transmission and can stop an outbreak.
Social distancing, also called physical distancing, is a set of non-pharmaceutical interventions or measures taken to prevent the spread of a contagious disease
by maintaining a physical distance between people and reducing the
number of times people come into close contact with each other.
It typically involves keeping a certain distance from others (the
distance specified may differ from time to time and country to country)
and avoiding gathering together in large groups.
By reducing the probability that a given uninfected person will come into physical contact with an infected person, the disease transmission can be suppressed, resulting in fewer deaths. The measures are used in combination with good respiratory hygiene and hand washing by a population. During the COVID-19 pandemic, the World Health Organization
(WHO) suggested favoring the term "physical distancing" as opposed to
"social distancing", in keeping with the fact that it is a physical
distance which prevents transmission; people can remain socially
connected via technology.
To slow down the spread of infectious diseases and avoid overburdening healthcare systems, particularly during a pandemic, several social-distancing measures are used, including the closing of schools and workplaces, isolation, quarantine, restricting the movement of people and the cancellation of mass gatherings.
Although the term was only introduced in the twenty-first century, social-distancing measures date back to at least the fifth century BC. The Bible contains one of the earliest known references to the practice in the Book of Leviticus 13:46: "And the leper in whom the plague is ... he shall dwell alone; [outside] the camp shall his habitation be." During the Plague of Justinian of 541 to 542, emperor Justinian enforced an ineffective quarantine on the Byzantine Empire, including dumping bodies into the sea; he predominantly blamed the widespread outbreak on "Jews, Samaritans, pagans, heretics, Arians, Montanists, and homosexuals". In modern times, social distancing measures have been successfully implemented in several epidemics. In St. Louis, shortly after the first cases of influenza were detected in the city during the 1918 flu pandemic,
authorities implemented school closures, bans on public gatherings and
other social-distancing interventions. The case fatality rates in
St. Louis were much less than in Philadelphia,
which despite having cases of influenza, allowed a mass parade to
continue and did not introduce social distancing until more than two
weeks after its first cases. Authorities have encouraged or mandated social distancing during the COVID-19 pandemic.
Social distancing measures are more effective when the infectious disease spreads via one or more of the following methods:
- droplet contact (coughing or sneezing)
- direct physical contact (including sexual contact)
- indirect physical contact (e.g., by touching a contaminated surface)
- airborne transmission (if the microorganism can survive in the air for long periods)
The measures are less effective when an infection is transmitted primarily via contaminated water or food or by vectors such as mosquitoes or other insects.
Drawbacks of social distancing can include loneliness, reduced productivity and the loss of other benefits associated with human interaction.
Definition
A poster (in Arabic, English and Urdu) encouraging social distancing during the COVID-19 pandemic
The Centers for Disease Control and Prevention
(CDC) have described social distancing as a set of "methods for
reducing frequency and closeness of contact between people in order to
decrease the risk of transmission of disease". During the 2009 flu pandemic the WHO described social distancing as "keeping at least an arm's length distance from others, [and] minimizing gatherings". It is combined with good respiratory hygiene and hand washing, and is considered the most feasible way to reduce or delay a pandemic.
During the COVID-19 pandemic, the CDC revised the definition of
social distancing as "remaining out of congregrate settings, avoiding
mass gatherings, and maintaining distance (approximately six feet or two
meters) from others when possible".
It is not clear why six feet was chosen. Recent studies have suggested
that droplets from a sneeze or forceful breathing during exercise can
travel over six meters. Some have suggested the required distance is based on debunked research from the 1930s and 1940s or confusion regarding units of measurement. Researchers and science writers have recommended that larger social distances and/or both mask wearing and social distancing be required.
Measures
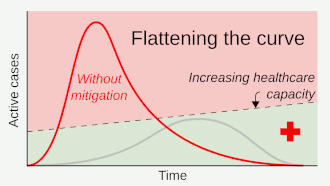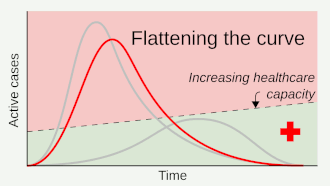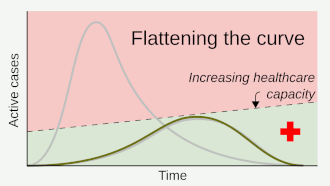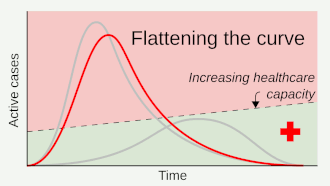
Social distancing helps prevent a sharp peak of infections ("flattens the epidemic curve") to help healthcare services deal with demand, and extends time for healthcare services to be increased and improved.
Knowing that a disease is circulating may trigger a change in behavior
by people choosing to stay away from public places and other people.
When implemented to control epidemics, such social distancing can result
in benefits but with an economic cost. Research indicates that measures
must be applied rigorously and immediately in order to be effective. Several social distancing measures are used to control the spread of contagious illnesses.
Avoiding physical contact
Keeping at least two-metre (six-foot) distance (in the US or UK) or
1.5 metres distance (in Australia) or 1 metre distance (in France or
Italy) from each other and avoiding hugs and gestures that involve direct physical contact, reduce the risk of becoming infected during flu pandemics and the coronavirus pandemic of 2020. These distances of separation, in addition to personal hygiene measures, are also recommended at places of work. Where possible it may be recommended to work from home.
Various alternatives have been proposed for the tradition of handshaking. The gesture of namaste,
placing one's palms together, fingers pointing upwards, drawing the
hands to the heart, is one non-touch alternative. During the COVID-19 pandemic in the United Kingdom, this gesture was used by Prince Charles upon greeting reception guests, and has been recommended by the Director-General of the WHO, Tedros Adhanom Ghebreyesus, and Israeli Prime Minister Benjamin Netanyahu. Other alternatives include the wave, the shaka (or "hang loose") sign, and placing a palm on your heart, as practiced in parts of Iran.
School closures
Swine flu cases per week in the United Kingdom in 2009; schools typically close for summer in mid-July and re-open in early September.
Mathematical modeling has shown that transmission of an outbreak may
be delayed by closing schools. However, effectiveness depends on the
contacts children maintain outside of school. Often, one parent has to
take time off work, and prolonged closures may be required. These
factors could result in social and economic disruption.
Workplace closures
Modeling and simulation
studies based on U.S. data suggest that if 10% of affected workplaces
are closed, the overall infection transmission rate is around 11.9% and
the epidemic peak time is slightly delayed. In contrast, if 33% of
affected workplaces are closed, the attack rate decreases to 4.9%, and
the peak time is delayed by one week.
Workplace closures include closure of "non-essential" businesses and
social services ("non-essential" means those facilities that do not
maintain primary functions in the community, as opposed to essential services).
Canceling mass gatherings
Cancellation of mass gatherings includes sports events, films or musical shows. Evidence suggesting that mass gatherings increase the potential for infectious disease transmission is inconclusive. Anecdotal evidence suggests certain types of mass gatherings may be associated with increased risk of influenza transmission,
and may also "seed" new strains into an area, instigating community
transmission in a pandemic. During the 1918 influenza pandemic, military parades in Philadelphia and Boston
may have been responsible for spreading the disease by mixing infected
sailors with crowds of civilians. Restricting mass gatherings, in
combination with other social distancing interventions, may help reduce
transmission.
Travel restrictions
Border
restrictions or internal travel restrictions are unlikely to delay an
epidemic by more than two to three weeks unless implemented with over
99% coverage. Airport screening was found to be ineffective in preventing viral transmission during the 2003 SARS outbreak in Canada and the U.S. Strict border controls between Austria and the Ottoman Empire, imposed from 1770 until 1871 to prevent persons infected with the bubonic plague
from entering Austria, were reportedly effective, as there were no
major outbreaks of plague in Austrian territory after they were
established, whereas the Ottoman Empire continued to suffer frequent
epidemics of plague until the mid-nineteenth century.
A Northeastern University study published in March 2020 found that "travel restrictions to and from China only slow down the international spread of COVID-19
[when] combined with efforts to reduce transmission on a community and
an individual level. [...] Travel restrictions aren't enough unless we
couple it with social distancing."
The study found that the travel ban in Wuhan delayed the spread of the
disease to other parts of mainland China only by three to five days,
although it did reduce the spread of international cases by as much as
80 percent. A primary reason travel restrictions were less effective is
that many people with COVID-19 do not show symptoms during the early
stages of infection.
Shielding
Social distancing markers and plexiglass shield at Whole Foods Market checkout in Toronto to reduce physical contact.
Shielding measures for individuals include limiting face-to-face
contacts, conducting business by phone or online, avoiding public places
and reducing unnecessary travel.
Quarantine
During the 2003 SARS outbreak in Singapore, approximately 8000 people were subjected to mandatory home quarantine and an additional 4300
were required to self-monitor for symptoms and make daily telephone
contact with health authorities as a means of controlling the epidemic.
Although only 58 of these individuals were eventually diagnosed with
SARS, public health officials were satisfied that this measure assisted
in preventing further spread of the infection. Voluntary self-isolation may have helped reduce transmission of influenza in Texas in 2009. Short and longterm negative psychological effects have been reported.
Stay-at-home orders
The objective of stay-at-home orders is to reduce day-to-day contact with between people and thereby reduce spread of infection
Cordon sanitaire
In 1995, a cordon sanitaire was used to control an outbreak of Ebola virus disease in Kikwit, Zaire. President Mobutu Sese Seko surrounded the town with troops and suspended all flights into the community. Inside Kikwit, the World Health Organization and Zaire's medical teams erected further cordons sanitaires, isolating burial and treatment zones from the general population and successfully containing the infection.
Protective sequestration
During the 1918 influenza epidemic, the town of Gunnison,
Colorado, isolated itself for two months to prevent an introduction of
the infection. Highways were barricaded and arriving train passengers
were quarantined for five days. As a result of the isolation, no one
died of influenza in Gunnison during the epidemic. Several other communities adopted similar measures.
Other measures
Other measures include shutting down or limiting mass transit and closure of sport facilities (community swimming pools, youth clubs, gymnasiums).
History
Leper colonies and lazarettos were established as a means of preventing the spread of leprosy and other contagious diseases through social distancing, until transmission was understood and effective treatments invented.
- The Lazzaretto of Ancona was constructed in the 18th century on an artificial island to serve as a quarantine station and leprosarium for the port town of Ancona, Italy.
- Two lepers denied entrance to town. Woodcut by Vincent of Beauvais, 14th century
- Passenger without mask being refused boarding of a streetcar amid 1918 flu pandemic. (Seattle, Washington, 1918)
1916 New York City polio epidemic
During the 1916 New York City polio epidemic,
when there were more than 27,000 cases and more than 6,000 deaths due
to polio in the United States, with more than 2,000 deaths in New York
City alone, movie theatres were closed, meetings were cancelled, public
gatherings were almost non-existent, and children were warned not to
drink from water fountains, and told to avoid amusement parks, swimming
pools and beaches.
Influenza, 1918 to present
During the influenza pandemic of 1918, Philadelphia saw its first cases of influenza on 17 September. The city continued with its planned parade and gathering of more than 200000 people and over the subsequent three days, the city's 31 hospitals became fully occupied. Over one week, 4500 people died. Social distancing measures were introduced on 3 October, on the orders of St. Louis physician Max C. Starkloff, more than two weeks after the first case. Unlike Philadelphia, St. Louis experienced its first cases of influenza on 5 October and the city took two days to implement several social distancing measures,
including closing schools, theatres, and other places where people get
together. It banned public gatherings, including funerals. The actions
slowed the spread of influenza in St. Louis and a spike in cases and
deaths, as had happened in Philadelphia, did not occur. The final death rate in St. Louis increased following a second wave of cases, but remained overall less than in other cities. Bootsma and Ferguson
analyzed social distancing interventions in sixteen U.S. cities during
the 1918 epidemic and found that time-limited interventions reduced
total mortality only moderately (perhaps 10–30%), and that the impact
was often very limited because the interventions were introduced too
late and lifted too early. It was observed that several cities
experienced a second epidemic peak after social distancing controls were
lifted, because susceptible individuals who had been protected were now
exposed.
School closures were shown to reduce morbidity from the Asian flu by 90% during the 1957–1958 pandemic, and up to 50% in controlling influenza in the U.S., 2004–2008.
Similarly, mandatory school closures and other social distancing
measures were associated with a 29% to 37% reduction in influenza
transmission rates during the 2009 flu epidemic in Mexico.
During the swine flu outbreak in 2009 in the UK, in an article titled "Closure of schools during an influenza pandemic" published in The Lancet Infectious Diseases,
a group of epidemiologists endorsed the closure of schools in order to
interrupt the course of the infection, slow further spread and buy time
to research and produce a vaccine. Having studied previous influenza pandemics including the 1918 flu pandemic, the influenza pandemic of 1957 and the 1968 flu pandemic,
they reported on the economic and workforce effect school closure would
have, particularly with a large percentage of doctors and nurses being
women, of whom half had children under the age of 16. They also looked
at the dynamics of the spread of influenza in France during French
school holidays and noted that cases of flu dropped when schools closed
and re-emerged when they re-opened. They noted that when teachers in
Israel went on strike during the flu season of 1999–2000, visits to
doctors and the number of respiratory infections dropped by more than a
fifth and more than two fifths respectively.
SARS 2003
During the SARS outbreak of 2003,
social distancing measures such as banning large gatherings, closing
schools and theaters, and other public places, supplemented public
health measures such as finding and isolating affected people,
quarantining their close contacts, and infection control procedures.
This was combined with wearing masks for certain people. During this time in Canada, "community quarantine" was used to reduce transmission of the disease with moderate success.
COVID-19 pandemic
Simulations
comparing rate of spread of infection, and number of deaths due to
overrun of hospital capacity, when social interactions are "normal"
(left, 200 people moving freely) and "distanced" (right, 25 people
moving freely).
Green = Healthy, uninfected individuals
Red = Infected individuals
Blue = Recovered individual
Black = Dead individuals
Green = Healthy, uninfected individuals
Red = Infected individuals
Blue = Recovered individual
Black = Dead individuals
During the COVID-19 pandemic,
social distancing and related measures are emphasised by several
governments as alternatives to an enforced quarantine of heavily
affected areas. According to UNESCO monitoring, more than a hundred countries have implemented nationwide school closures in response to COVID-19, impacting over half the world's student population.
In the United Kingdom, the government advised the public to avoid
public spaces, and cinemas and theatres voluntarily closed to encourage
the government's message.
With many people disbelieving that COVID-19 is any worse than the seasonal flu,
it has been difficult to convince the public—especially teens and young
adults—to voluntarily adopt social distancing practices. In Belgium, media reported a rave was attended by at least 300 before it was broken up by local authorities. In France teens making nonessential trips are fined up to US$150. Beaches were closed in Florida and Alabama to disperse partygoers during spring break. Weddings were broken up in New Jersey and an 8 p.m. Curfew was imposed in Newark.
New York, New Jersey, Connecticut and Pennsylvania were the first
states to adopt coordinated social distancing policies which closed down
non-essential businesses and restricted large gatherings. Shelter in
place orders in California were extended to the entire state on 19 March. On the same day Texas declared a public disaster and imposed statewide restrictions.
These preventive measures such as social-distancing and self-isolation prompted the widespread closure of primary, secondary, and post-secondary schools in more than 120 countries. As of 23 March 2020, more than 1.2 billion learners were out of school due to school closures in response to COVID-19. Given low rates of COVID-19 symptoms among children, the effectiveness of school closures has been called into question. Even when school closures are temporary, it carries high social and economic costs. However, the significance of children in spreading COVID-19 is unclear.
While the full impact of school closures during the coronavirus
pandemic are not yet known, UNESCO advises that school closures have
negative impacts on local economies and on learning outcomes for
students.
In early March 2020, the sentiment "Stay The Fuck Home" was
coined by Florian Reifschneider, a German engineer and was quickly
echoed by notable celebrities such as Taylor Swift, Ariana Grande and Busy Philipps in hopes of reducing and delaying the peak of the outbreak. Facebook, Twitter and Instagram
also joined the campaign with similar hashtags, stickers and filters
under #staythefhome, #stayhome, #staythefuckhome and began trending
across social media. The website claims to have reached about two million people online and says the text has been translated into 17 languages.
Drawbacks
There are concerns that social distancing can have adverse affects on participants' mental health. It may lead to stress, anxiety, depression or panic, especially for individuals with preexisting conditions such as anxiety disorders, obsessive compulsive disorders, and paranoia.
Widespread media coverage about a pandemic, its impact on economy, and
resulting hardships may create anxiety. Change in daily circumstances
and uncertainty about the future may add onto the mental stress of being
away from other people.
Portrayal in literature
In his 1957 science fiction novel The Naked Sun, Isaac Asimov
portrays a planet where people live with social distancing. They are
spread out, miles from each other, across a sparsely-populated world.
Communication is primarily through technology. A male and a female still
need to engage in sex to make a baby, but because of the risk of
disease transmission it is a dangerous, nasty chore. In contrast, when
communication is through technology the situation is the reverse: there
is no modesty, and casual nudity is frequent. The novel's point of
departure is a murder: this seemingly idyllic world in fact has serious
social problems.
Theoretical basis
A look at the math behind social distancing amid coronavirus where the goal is to decrease the effective reproduction number, , which starts off equal to ,
the basic reproduction number, which is the average number of secondary
infected individuals generated from one primary infected individual in a
population where all individuals are equally susceptible to COVID-19
From the perspective of epidemiology, the basic goal behind social distancing is to decrease the effective reproduction number, or , which in the absence of social distancing would equate to the basic reproduction number,
i.e. the average number of secondary infected individuals generated
from one primary infected individual in a population where all
individuals are equally susceptible to a disease. In a basic model of
social distancing, where a proportion of the population engages in social distancing to decrease their interpersonal contacts to a fraction of their normal contacts, the new effective reproduction number is given by:
For example, 25% of the population reducing their social
contacts to 50% of their normal level gives an effective reproduction
number about 81% of the basic reproduction number. A seemingly small
reduction has a statistically significant effect in delaying the
exponential growth and spread of a disease.
Where the value of can be brought below 1 for sufficiently long, containment is achieved, and the number infected should decrease.



















![{\displaystyle R=[1-(1-a^{2})f]R_{0}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/94c687110c77a2fe49b9f726d449812b8a517df4)