From Wikipedia, the free encyclopedia
Sucrose

|
|
|
| Names
|
| IUPAC name
β-D-Fructofuranosyl α-D-glucopyranoside
|
Systematic IUPAC name
(2R,3R,4S,5S,6R)-2-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol
|
Other names
- Sugar;
- Saccharose;
- α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside;
- β-D-fructofuranosyl-(2→1)-α-D-glucopyranoside;
- β-(2S,3S,4S,5R)-fructofuranosyl-α-(1R,2R,3S,4S,5R)-glucopyranoside;
- α-(1R,2R,3S,4S,5R)-glucopyranosyl-β-(2S,3S,4S,5R)-fructofuranoside;
- Dodecacarbon monodecahydrate;
- ((2R,3R,4S,5S,6R)-2-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxapent-2-yl]oxy-6-(hydroxymethyl)oxahexane-3,4,5-triol)
|
| Identifiers
|
|
|
|
|
|
|
| ChEBI
|
|
| ChEMBL
|
|
| ChemSpider
|
|
| DrugBank
|
|
| ECHA InfoCard
|
100.000.304
|
| EC Number
|
200-334-9
|
|
|
|
|
|
|
| RTECS number
|
WN6500000
|
| UNII
|
|
|
|
|
|
|
|
|
| Properties
|
|
|
C12H22O11
|
| Molar mass
|
342.30 g/mol
|
| Appearance
|
white solid
|
| Density
|
1.587 g/cm3, solid
|
| Melting point
|
None; decomposes at 186 °C (367 °F; 459 K)
|
|
|
~200 g/dL (25 °C) |
| log P
|
−3.76
|
| Structure
|
|
|
Monoclinic
|
|
|
P21
|
| Thermochemistry
|
|
|
1,349.6 kcal/mol (5,647 kJ/mol) (Higher heating value)
|
| Hazards
|
| Safety data sheet
|
ICSC 1507
|
| NFPA 704
|
|
| Lethal dose or concentration (LD, LC):
|
|
|
29700 mg/kg (oral, rat)
|
| US health exposure limits (NIOSH):
|
|
|
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)
|
|
|
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)
|
|
|
N.D.
|
| Related compounds
|
Related compounds
|
Lactose
Maltose
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
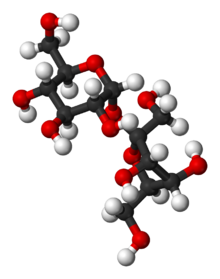
Sucrose is common
sugar. It is a
disaccharide, a molecule composed of two
monosaccharides:
glucose and
fructose. Sucrose is produced naturally in plants, from which
table sugar is refined. It has the
molecular formula C
12H
22O
11.
For human consumption, sucrose is extracted, and refined, from either
sugar cane or
sugar beet.
Sugar mills are located where sugarcane is grown to crush the cane and
produce raw sugar which is shipped around the world for refining into
pure sucrose. Some sugar mills also process the raw sugar into pure
sucrose. Sugar beet factories are located in colder climates where the
beet is grown and process the beets directly into refined sugar. The
sugar refining process involves washing the raw sugar crystals before
dissolving them into a sugar syrup which is filtered and then passed
over carbon to remove any residual colour. The by-now clear sugar syrup
is then concentrated by boiling under a vacuum and crystallized as the
final purification process to produce crystals of pure sucrose. These
crystals are clear, odourless, and have a sweet taste. En masse, the
crystals appear white.
Sugar is often an added ingredient in food production and food
recipes. About 185 million
tonnes of sugar were produced worldwide in 2017.
Etymology
The word
sucrose was coined in 1857 by the English chemist
William Miller from the French
sucre ("sugar") and the generic chemical suffix for sugars
-ose. The abbreviated term
Suc is often used for
sucrose in scientific literature.
The name
saccharose was coined in 1860 by the French chemist
Marcellin Berthelot. Saccharose is an obsolete name for sugars in general, especially sucrose.
Physical and chemical properties
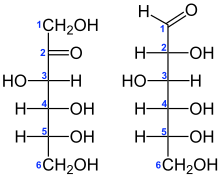
Structural O-α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside
In sucrose, the components glucose and fructose are linked via an ether bond between C1 on the
glucosyl subunit and C2 on the fructosyl unit. The bond is called a
glycosidic linkage.
Glucose exists predominantly as two isomeric "pyranoses" (α and β), but
only one of these forms links to the fructose. Fructose itself exists
as a mixture of "furanoses", each of which having α and β isomers, but
only one particular isomer links to the glucosyl unit. What is notable
about sucrose is that, unlike most disaccharides, the glycosidic bond is
formed between the reducing ends of both glucose and fructose, and not
between the reducing end of one and the nonreducing end of the other.
This linkage inhibits further bonding to other saccharide units. Since
it contains no anomeric hydroxyl groups, it is classified as a non-
reducing sugar.
Sucrose crystallizes in the
monoclinic space group P2
1 with room-temperature lattice parameters
a = 1.08631 nm,
b = 0.87044 nm,
c = 0.77624 nm, β = 102.938°.
The purity of sucrose is measured by
polarimetry, through the rotation of
plane-polarized light by a solution of sugar. The
specific rotation
at 20 °C using yellow "sodium-D" light (589 nm) is +66.47°. Commercial
samples of sugar are assayed using this parameter. Sucrose does not
deteriorate at ambient conditions.
Thermal and oxidative degradation
- C12H22O11 + 6 KNO3 → 9 CO + 3 N2 + 11 H2O + 3 K2CO3
This reaction is somewhat simplified though. Some of the carbon does
get fully oxidized to carbon dioxide, and other reactions, such as the
water-gas shift reaction also take place. A more accurate theoretical equation is:
- C12H22O11 + 6.288 KNO3 → 3.796 CO2 + 5.205 CO + 7.794 H2O + 3.065 H2 + 3.143 N2 + 2.998 K2CO3 + 0.274 KOH
- 8 HClO3 + C12H22O11 → 11 H2O + 12 CO2 + 8 HCl
Sucrose can be dehydrated with
sulfuric acid to form a black,
carbon-rich solid, as indicated in the following idealized equation:
- H2SO4(catalyst) + C12H22O11 → 12 C + 11 H2O + Heat (and some H2O + SO3 as a result of the heat).
The formula for sucrose's decomposition can be represented as a
two-step reaction: the first simplified reaction is dehydration of
sucrose to pure carbon and water, and then carbon oxidises to CO2 with O2 from air.
- C12H22O11 + heat → 12 C + 11 H2O
- 12 C + 12 O2 → 12 CO2
Hydrolysis
Hydrolysis breaks the
glycosidic bond converting sucrose into
glucose and
fructose.
Hydrolysis is, however, so slow that solutions of sucrose can sit for years with negligible change. If the
enzyme sucrase is added, however, the reaction will proceed rapidly. Hydrolysis can also be accelerated with acids, such as
cream of tartar
or lemon juice, both weak acids. Likewise, gastric acidity converts
sucrose to glucose and fructose during digestion, the bond between them
being an acetal bond which can be broken by an acid.
Given
(higher) heats of combustion of 1349.6 kcal/mol for sucrose, 673.0 for glucose, and 675.6 for fructose, hydrolysis releases about 1.0 kcal (4.2 kJ) per mole of sucrose, or about 3
small calories per gram of product.
Synthesis and biosynthesis of sucrose
Chemical synthesis
Model of sucrose molecule
Although sucrose is almost invariably isolated from natural sources, its chemical synthesis was first achieved in 1953 by
Raymond Lemieux.
Sources
In nature, sucrose is present in many plants, and in particular their roots, fruits and
nectars, because it serves as a way to store energy, primarily from
photosynthesis.
Many mammals, birds, insects and bacteria accumulate and feed on the
sucrose in plants and for some it is their main food source. Seen from a
human consumption perspective,
honeybees are especially important because they accumulate sucrose and produce
honey, an important foodstuff all over the world. The carbohydrates in honey itself primarily consist of
fructose and
glucose with trace amounts of sucrose only.
As fruits ripen, their sucrose content usually rises sharply, but
some fruits contain almost no sucrose at all. This includes grapes,
cherries, blueberries, blackberries, figs, pomegranates, tomatoes,
avocados, lemons and limes.
Sucrose is a naturally occurring sugar, but with the advent of
industrialization, it has been increasingly refined and consumed in all kinds of processed foods.
Production
History of sucrose refinement
Table sugar production in the 19th century. Sugar cane
plantations (upper image) employed slave or indentured laborers. The
picture shows workers harvesting cane, loading it on a boat for
transport to the plant, while a European overseer watches in the lower
right. The lower image shows a sugar plant with two furnace chimneys.
Sugar plants and plantations were harsh, inhumane work.
A sugarloaf was a traditional form for sugar from the 17th to 19th centuries. Sugar nips were required to break off pieces.
The production of table sugar has a long history. Some scholars claim Indians discovered how to crystallize sugar during the
Gupta dynasty, around AD 350.
Other scholars point to the ancient manuscripts of China, dated
to the 8th century BC, where one of the earliest historical mentions of
sugar cane is included along with the fact that their knowledge of sugar cane was derived from India.
Further, it appears that by about 500 BC, residents of present-day
India began making sugar syrup and cooling it in large flat bowls to
make raw table sugar crystals that were easier to store and transport.
In the local Indian language, these crystals were called
khanda (खण्ड), which is the source of the word
candy.
The army of Alexander the Great was halted on the banks of river
Indus by the refusal of his troops to go further east. They saw people in the Indian subcontinent growing sugarcane and making
granulated, salt-like sweet powder, locally called
sākhar (साखर), pronounced as
sakcharon (ζακχαρον) in Greek (Modern Greek,
zachari
ζάχαρη). On their return journey, the Greek soldiers carried back some
of the "honey-bearing reeds". Sugarcane remained a limited crop for over
a millennium. Sugar was a rare commodity and traders of sugar became
wealthy. Venice, at the height of its financial power, was the chief
sugar-distributing center of Europe. Arabs started producing it in
Sicily and
Spain. Only after the
Crusades did it begin to rival honey as a sweetener in Europe. The Spanish began cultivating sugarcane in the
West Indies in 1506 (
Cuba in 1523). The
Portuguese first cultivated sugarcane in
Brazil in 1532.
Sugar remained a luxury in much of the world until the 18th
century. Only the wealthy could afford it. In the 18th century, the
demand for table sugar boomed in Europe and by the 19th century it had
become regarded as a human necessity. The use of sugar grew from use in tea, to
cakes,
confectionery and
chocolates. Suppliers marketed sugar in novel forms, such as solid cones, which required consumers to use a
sugar nip, a pliers-like tool, in order to break off pieces.
The demand for cheaper table sugar drove, in part, colonization
of tropical islands and nations where labor-intensive sugarcane
plantations and table sugar manufacturing could thrive. Growing sugar
cane crop in hot humid climates, and producing table sugar in high
temperature sugar mills was harsh, inhumane work. The demand for cheap
and docile labor for this work, in part, first drove slave trade from
Africa (in particular West Africa), followed by indentured labor trade
from South Asia (in particular India).
Millions of slaves, followed by millions of indentured laborers were
brought into the Caribbean, Indian Ocean, Pacific Islands, East Africa,
Natal, north and eastern parts of South America, and southeast Asia. The
modern ethnic mix of many nations, settled in the last two centuries,
has been influenced by table sugar.
Beginning in the late 18th century, the production of sugar became increasingly mechanized. The
steam engine first powered a sugar mill in
Jamaica
in 1768, and, soon after, steam replaced direct firing as the source of
process heat. During the same century, Europeans began experimenting
with sugar production from other crops.
Andreas Marggraf identified sucrose in
beet root and his student
Franz Achard built a sugar beet processing factory in Silesia (Prussia). The beet-sugar industry took off during the
Napoleonic Wars,
when France and the continent were cut off from Caribbean sugar. In
2010, about 20 percent of the world's sugar was produced from beets.
Today, a large beet refinery producing around 1,500 tonnes of
sugar a day needs a permanent workforce of about 150 for 24-hour
production.
Trends
A table sugar factory in England. The tall diffusers
are visible to the middle left where the harvest transforms into a
sugar syrup. The boiler and furnace are in the center, where table sugar
crystals form. An expressway for transport is visible in the lower
left.
Table sugar (sucrose) comes from plant sources. Two important sugar crops predominate:
sugarcane (
Saccharum spp.) and
sugar beets (
Beta vulgaris), in which sugar can account for 12% to 20% of the plant's dry weight. Minor commercial sugar crops include the
date palm (
Phoenix dactylifera),
sorghum (
Sorghum vulgare), and the
sugar maple (
Acer saccharum).
Sucrose is obtained by extraction of these crops with hot water;
concentration of the extract gives syrups, from which solid sucrose can
be crystallized. In 2017, worldwide production of table sugar amounted
to 185 million tonnes.
Most cane sugar comes from countries with warm climates, because
sugarcane does not tolerate frost. Sugar beets, on the other hand, grow
only in cooler temperate regions and do not tolerate extreme heat. About
80 percent of sucrose is derived from sugarcane, the rest almost all
from sugar beets.
In mid-2018, India and Brazil had about the same production of sugar – 34 million tonnes – followed by the
European Union,
Thailand, and China as the major producers. India, the European Union, and China were the leading domestic consumers of sugar in 2018.
Beet sugar comes from regions with cooler climates: northwest and
eastern Europe, northern Japan, plus some areas in the United States
(including California). In the northern hemisphere, the beet-growing
season ends with the start of harvesting around September. Harvesting
and processing continues until March in some cases. The availability of
processing plant capacity and the weather both influence the duration of
harvesting and processing – the industry can store harvested beets
until processed, but a frost-damaged beet becomes effectively
unprocessable.
The United States sets high sugar prices to support its
producers, with the effect that many former purchasers of sugar have
switched to
corn syrup (beverage manufacturers) or moved out of the country (candy manufacturers).
High-fructose corn syrup
In the United States, there are tariffs on the importation of sugar, and subsidies for the production of
maize
(corn). High-fructose corn syrup (HFCS) is significantly cheaper than
refined sucrose as a sweetener. This has led to sucrose being partially
displaced in U.S. industrial food production by HFCS and other
non-sucrose natural sweeteners.
Some people regard HFCS as unhealthy. Clinical nutritionists, medical authorities, and the United States
Food and Drug Administration
have dismissed such concerns because "Sucrose, HFCS, invert sugar,
honey, and many fruits and juices deliver the same sugars in the same
ratios to the same tissues within the same time frame to the same
metabolic pathways". While scientific authorities agree that dietary sugars are a source of
empty calories
associated with certain health problems, the belief that
glucose-fructose syrups such as HFCS are especially unhealthy is not
supported by scientific evidence. The FDA does endorse limiting the
consumption of all added sugars, including HFCS.
Types
Cane
Harvested sugarcane from Venezuela ready for processing
Since the 6th century BC, cane sugar producers have crushed the
harvested vegetable material from sugarcane in order to collect and
filter the juice. They then treat the liquid (often with
lime (calcium oxide))
to remove impurities and then neutralize it. Boiling the juice then
allows the sediment to settle to the bottom for dredging out, while the
scum rises to the surface for skimming off. In cooling, the liquid
crystallizes, usually in the process of stirring, to produce sugar
crystals.
Centrifuges
usually remove the uncrystallized syrup. The producers can then either
sell the sugar product for use as is, or process it further to produce
lighter grades. The later processing may take place in another factory
in another country.
Sugarcane is a major component of Brazilian agriculture; the
country is the world's largest producer of sugarcane and its derivative
products, such as crystallized sugar and
ethanol (
ethanol fuel).
Beet
Beet sugar producers slice the washed beets, then extract the sugar with hot water in a "
diffuser". An alkaline solution ("
milk of lime" and
carbon dioxide from the lime kiln) then serves to
precipitate impurities (see
carbonatation). After filtration,
evaporation concentrates the juice to a content of about 70% solids,
and controlled crystallisation extracts the sugar. A centrifuge removes
the sugar crystals from the liquid, which gets recycled in the
crystalliser stages. When economic constraints prevent the removal of
more sugar, the manufacturer discards the remaining liquid, now known as
molasses, or sells it on to producers of animal feed.
Sieving the resultant white sugar produces different grades for selling.
Cane versus beet
Sugar cane tolerates hot climates better, but the production of
sugar cane needs approximately four times as much water as the
production of sugar beet. As a result, some countries that traditionally
produced cane sugar (such as
Egypt)
have built new beet sugar factories since about 2008. Some sugar
factories process both sugar cane and sugar beets and extend their
processing period in that way.
The production of sugar leaves residues that differ substantially
depending on the raw materials used and on the place of production.
While cane
molasses
is often used in food preparation, humans find molasses from sugar
beets unpalatable, and it consequently ends up mostly as industrial
fermentation feedstock (for example in
alcohol distilleries), or as
animal feed. Once dried, either type of molasses can serve as fuel for burning.
Pure beet sugar is difficult to find, so labelled, in the
marketplace. Although some makers label their product clearly as "pure
cane sugar", beet sugar is almost always labeled simply as sugar or pure
sugar. Interviews with the 5 major beet sugar-producing companies
revealed that many store brands or "private label" sugar products are
pure beet sugar. The lot code can be used to identify the company and
the plant from which the sugar came, enabling beet sugar to be
identified if the codes are known.
Culinary sugars
Mill white
Mill white, also called plantation white, crystal sugar or superior sugar is produced from raw sugar. It is exposed to
sulfur dioxide
during the production to reduce the concentration of color compounds
and helps prevent further color development during the crystallization
process. Although common to sugarcane-growing areas, this product does
not store or ship well. After a few weeks, its impurities tend to
promote discoloration and clumping; therefore this type of sugar is
generally limited to local consumption.
Blanco directo
Blanco
directo, a white sugar common in India and other south Asian countries,
is produced by precipitating many impurities out of cane juice using
phosphoric acid and
calcium hydroxide, similar to the
carbonatation technique used in beet sugar refining. Blanco directo is more pure than mill white sugar, but less pure than white refined.
White refined
White refined is the most common form of sugar in North America and
Europe. Refined sugar is made by dissolving and purifying raw sugar
using
phosphoric acid similar to the method used for blanco directo, a
carbonatation
process involving calcium hydroxide and carbon dioxide, or by various
filtration strategies. It is then further purified by filtration through
a bed of
activated carbon or
bone char. Beet sugar refineries produce refined white sugar directly without an intermediate raw stage.
White refined sugar is typically sold as granulated sugar, which has been dried to prevent clumping and comes in various crystal sizes for home and industrial use:
Sugars; clockwise from top left: Refined, unrefined, brown, unprocessed cane
- Coarse-grain, such as sanding sugar (also called "pearl sugar", "decorating sugar", nibbed sugar or sugar nibs)
is a coarse grain sugar used to add sparkle and flavor atop baked goods
and candies. Its large reflective crystals will not dissolve when
subjected to heat.
- Granulated, familiar as table sugar, with a grain size about 0.5 mm across. "Sugar cubes" are lumps for convenient consumption produced by mixing granulated sugar with sugar syrup.
- Caster (or castor) (0.35 mm), a very fine sugar in Britain and other Commonwealth countries, so-named because the grains are small enough to fit through a castor which is small vessel with a perforated top, from which to sprinkle sugar at table. Commonly used in baking and mixed drinks, it is sold as "superfine"
sugar in the United States. Because of its fineness, it dissolves
faster than regular white sugar and is especially useful in meringues
and cold liquids. Castor sugar can be prepared at home by grinding
granulated sugar for a couple of minutes in a mortar or food processor.
- Powdered, 10X sugar, confectioner's sugar (0.060 mm), or icing sugar (0.024 mm), produced by grinding sugar to a fine powder. The manufacturer may add a small amount of anticaking agent to prevent clumping — either cornstarch (1% to 3%) or tri-calcium phosphate.
Brown sugar comes either from the late stages of cane sugar refining, when sugar forms fine crystals with significant
molasses
content, or from coating white refined sugar with a cane molasses syrup
(blackstrap molasses). Brown sugar's color and taste becomes stronger
with increasing molasses content, as do its moisture-retaining
properties. Brown sugars also tend to harden if exposed to the
atmosphere, although proper handling can reverse this.
Measurement
Dissolved sugar content
Scientists and the
sugar industry use degrees
Brix (symbol °Bx), introduced by
Adolf Brix,
as units of measurement of the mass ratio of dissolved substance to
water in a liquid. A 25 °Bx sucrose solution has 25 grams of sucrose per
100 grams of liquid; or, to put it another way, 25 grams of sucrose
sugar and 75 grams of water exist in the 100 grams of solution.
The Brix degrees are measured using an infrared sensor. This
measurement does not equate to Brix degrees from a density or refractive
index measurement, because it will specifically measure dissolved sugar
concentration instead of all dissolved solids. When using a
refractometer, one should report the result as "
refractometric dried substance" (RDS). One might speak of a liquid as having 20 °Bx RDS. This refers to a measure of percent by weight of
total
dried solids and, although not technically the same as Brix degrees
determined through an infrared method, renders an accurate measurement
of sucrose content, since sucrose in fact forms the majority of dried
solids. The advent of in-line infrared Brix measurement sensors has made
measuring the amount of dissolved sugar in products economical using a
direct measurement.
Consumption
Refined sugar was a luxury before the 18th century. It became widely
popular in the 18th century, then graduated to becoming a necessary food
in the 19th century. This evolution of taste and demand for sugar as an
essential food ingredient unleashed major economic and social changes. Eventually, table sugar became sufficiently cheap and common enough to influence standard cuisine and flavored drinks.
Sucrose forms a major element in
confectionery and
desserts.
Cooks use it for sweetening — its fructose component, which has almost
double the sweetness of glucose, makes sucrose distinctively sweet in
comparison to other carbohydrates. It can also act as a
food preservative
when used in sufficient concentrations. Sucrose is important to the
structure of many foods, including biscuits and cookies, cakes and pies,
candy, and ice cream and sorbets. It is a common ingredient in many
processed and so-called "
junk foods".
Nutritional information
Metabolism of sucrose
Tooth decay
Tooth decay
(dental caries) has become a pronounced health hazard associated with
the consumption of sugars, especially sucrose. Oral bacteria such as
Streptococcus mutans live in dental plaque and metabolize
any sugars (not just sucrose, but also
glucose,
lactose,
fructose, and cooked
starches) into
lactic acid. The resultant lactic acid lowers the pH of the tooth's surface, stripping it of minerals in the process known as tooth decay.
All 6-carbon sugars and disaccharides based on 6-carbon sugars
can be converted by dental plaque bacteria into acid that demineralizes
teeth, but sucrose may be uniquely useful to
Streptococcus sanguinis (formerly
Streptococcus sanguis) and
Streptococcus mutans.
Sucrose is the only dietary sugar that can be converted to sticky
glucans (dextran-like polysaccharides) by extracellular enzymes. These
glucans allow the bacteria to adhere to the tooth surface and to build
up thick layers of plaque. The anaerobic conditions deep in the plaque
encourage the formation of acids, which leads to carious lesions. Thus,
sucrose could enable
S. mutans,
S. sanguinis and many
other species of bacteria to adhere strongly and resist natural removal,
e.g. by flow of saliva, although they are easily removed by brushing.
The glucans and levans (fructose polysaccharides) produced by the plaque
bacteria also act as a reserve food supply for the bacteria.
Such a special role of sucrose in the formation of tooth decay is much
more significant in light of the almost universal use of sucrose as the
most desirable sweetening agent. Widespread replacement of sucrose by
high-fructose corn syrup
(HFCS) has not diminished the danger from sucrose. If smaller amounts
of sucrose are present in the diet, they will still be sufficient for
the development of thick, anaerobic plaque and plaque bacteria will
metabolise other sugars in the diet, such as the glucose and fructose in HFCS.
Glycemic index
Sucrose is a
disaccharide made up of 50%
glucose and 50%
fructose and has a
glycemic index of 65. Sucrose is digested rapidly, but has a relatively low glycemic index due to its content of fructose, which has a minimal effect on blood glucose.
As with other sugars, sucrose is digested into its components via the enzyme
sucrase
to glucose (blood sugar) and fructose. The glucose component is
transported into the blood where it serves immediate metabolic demands,
or is converted and reserved in the
liver as
glycogen.
Gout
The occurrence of
gout
is connected with an excess production of uric acid. A diet rich in
sucrose may lead to gout as it raises the level of insulin, which
prevents excretion of uric acid from the body. As the concentration of
uric acid in the body increases, so does the concentration of uric acid
in the joint liquid and beyond a critical concentration, the uric acid
begins to precipitate into crystals. Researchers have implicated sugary
drinks high in fructose in a surge in cases of gout.
UN dietary recommendation
In 2015, the
World Health Organization (WHO)
published a new guideline on sugars intake for adults and children, as a
result of an extensive review of the available scientific evidence by a
multidisciplinary group of experts. The guideline recommends that both
adults and children reduce the intake of free sugars (monosaccharides
and disaccharides added to foods and beverages by the manufacturer, cook
or consumer, and sugars naturally present in honey, syrups, fruit
juices and fruit juice concentrates) to less than 10% of total energy
intake. A reduction to below 5% of total energy intake brings additional
health benefits, especially in what regards dental caries.
Religious concerns
The sugar refining industry often uses
bone char (
calcinated animal bones) for decolorizing. About 25% of sugar produced in the U.S. is processed using bone char as a filter, the remainder being processed with
activated carbon. As bone char does not seem to remain in finished sugar, Jewish religious leaders consider sugar filtered through it to be
pareve,
meaning that it is neither meat nor dairy and may be used with either
type of food. However, the bone char must source to a kosher animal
(e.g. cow, sheep) for the sugar to be
kosher.
Trade and economics
One
of the most widely-traded commodities in the world throughout history,
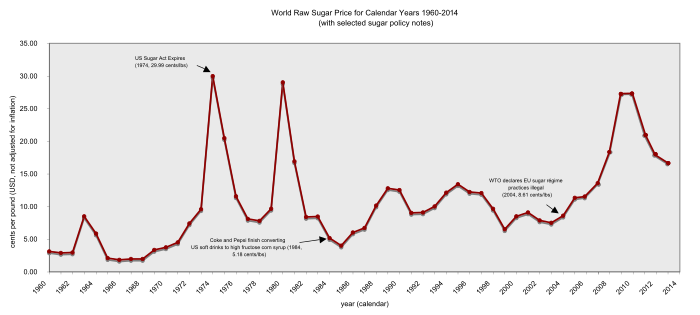
sugar accounts for around 2% of the global dry cargo market. International sugar prices show great volatility, ranging from around 3 to over 60 cents per pound in the past
50 years. About 100 of the world's 180 countries produce sugar from
beet or cane, a few more refine raw sugar to produce white sugar, and
all countries consume sugar. Consumption of sugar ranges from around 3
kilograms per person per annum in Ethiopia to around 40 kg/person/yr in
Belgium.
Consumption per capita rises with income per capita until it reaches a
plateau of around 35 kg per person per year in middle income countries.
Many countries subsidize sugar production heavily. The European
Union, the United States, Japan, and many developing countries subsidize
domestic production and maintain high tariffs on imports. Sugar prices
in these countries have often exceeded prices on the international
market by up to three times; today, with world market sugar futures prices currently strong, such prices typically exceed world prices by two times.
World raw sugar price from 1960 to 2014
Within international trade bodies, especially in the
World Trade Organization, the "
G20"
countries led by Brazil have long argued that, because these sugar
markets in essence exclude cane sugar imports, the G20 sugar producers
receive lower prices than they would under
free trade. While both the
European Union and United States maintain trade agreements whereby certain developing and
less developed countries
(LDCs) can sell certain quantities of sugar into their markets, free of
the usual import tariffs, countries outside these preferred trade
régimes have complained that these arrangements violate the "
most favoured nation" principle of international trade. This has led to numerous tariffs and levies in the past.
In 2004, the
WTO
sided with a group of cane sugar exporting nations (led by Brazil and
Australia) and ruled the EU sugar-régime and the accompanying ACP-EU
Sugar Protocol (whereby a group of African, Caribbean, and Pacific
countries receive preferential access to the European sugar market)
illegal.
In response to this and to other rulings of the WTO, and owing to
internal pressures on the EU sugar-régime, the European Commission
proposed on 22 June 2005 a radical reform of the EU sugar-régime,
cutting prices by 39% and eliminating all EU sugar exports.
The African, Caribbean, Pacific and
least developed country sugar exporters reacted with dismay to the EU sugar proposals. On 25 November 2005, the Council of the EU agreed to cut EU sugar prices by 36% as from 2009. In 2007, it seemed
that the
U.S. Sugar Program
could become the next target for reform. However, some commentators
expected heavy lobbying from the U.S. sugar industry, which donated $2.7
million to US House and US Senate incumbents in the 2006 US election,
more than any other group of US food-growers.
Especially prominent lobbyists include
The Fanjul Brothers, so-called "sugar barons" who made the single largest individual contributions of
soft money to both the Democratic and Republican parties in the political system of the United States of America.
Small quantities of sugar, especially specialty grades of sugar, reach the market as '
fair trade' commodities; the
fair trade
system produces and sells these products with the understanding that a
larger-than-usual fraction of the revenue will support small farmers in
the developing world. However, whilst the Fairtrade Foundation offers a
premium of $60.00 per tonne to small farmers for sugar branded as
"Fairtrade", government schemes such as the U.S. Sugar Program and the
ACP Sugar Protocol
offer premiums of around $400.00 per tonne above world market prices.
However, the EU announced on 14 September 2007 that it had offered "to
eliminate all duties and quotas on the import of sugar into the EU".
The
US Sugar Association has launched a campaign to promote sugar over artificial substitutes. The Association now
aggressively challenges many common beliefs regarding negative
side-effects of sugar consumption. The campaign aired a high-profile
television commercial during the 2007
Primetime Emmy Awards on FOX Television. The Sugar Association uses the trademark tagline "Sugar: sweet by nature".