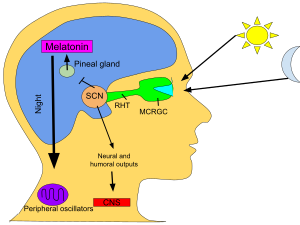
Melatonin is a
hormone that regulates the
sleep–wake cycle. It is primarily released by the
pineal gland. As a supplement, it is often used for the short-term treatment of
trouble sleeping such as from
jet lag or
shift work. Evidence of benefit, however, is unclear. One review found onset of sleep occurred 6 minutes faster with use but found no change in total time asleep. It may work as well as the medication
ramelteon. It is typically taken by mouth.
Side effects from supplements are minimal at low doses for short durations. Side effects may include sleepiness, headaches,
nausea,
diarrhea, and abnormal
dreams. Use is not recommended during
pregnancy or
breastfeeding. Use is also not recommended in those with
liver problems.
In animals (including humans), melatonin is involved in
synchronizing the
circadian rhythm including sleep–wake timing,
blood pressure regulation, and seasonal
reproduction. Many of its effects are through activation of the
melatonin receptors, while others are due to its role as an
antioxidant. In
plants it functions to defend against
oxidative stress. Melatonin is also present in various foods.
Melatonin was discovered in 1958. It is sold
over the counter in Canada and the United States. In the United Kingdom it is a prescription-only medication. A month's supply costs about US $1 to 4 in the United States. In the United Kingdom a month's supply costs the
NHS about 15 pounds. It is not
FDA-approved for any use. In Australia and
Europe, it is approved for trouble sleeping in people over the age of 54.
Medical use
Sleep disorders
Positions on the benefits of melatonin for
insomnia are mixed. An
Agency for Healthcare Research and Quality (AHRQ) review from 2015 stated that evidence of benefit in the general population was unclear. A review from 2017 found a modest effect on time until onset of sleep. Another review from 2017 put this decrease at 6 minutes to sleep onset but found no difference in total sleep time. Melatonin may also be useful in
delayed sleep phase syndrome. Melatonin appears to work as well as
ramelteon but costs less.
Dementia
A 2016 Cochrane review found no evidence that melatonin helped sleep problems in people with moderate to severe dementia due to
Alzheimer's disease.
A 2019 review found that while melatonin may improve sleep in minimal
cognitive impairment, after the onset of Alzheimer's it has little to no
effect. Melatonin may, however, help with sundowning.
Jet lag and shift work
Melatonin is known to reduce
jet lag, especially in eastward travel. If the time it is taken is not correct, however, it can instead delay adaption.
Melatonin appears to have limited use against the sleep problems of people who work
shift work. Tentative evidence suggests that it increases the length of time people are able to sleep.
Adverse effects
Melatonin appears to cause very few
side effects as tested in the short term, up to three months, at low doses.
Two systematic reviews found no adverse effects of exogenous melatonin
in several clinical trials and comparative trials found the adverse
effects headaches, dizziness, nausea, and drowsiness were reported about
equally for both melatonin and
placebo. Prolonged-release melatonin is safe with long-term use of up to 12 months.
Although not recommended for long term use beyond this, low-dose
melatonin is generally safer, and a better alternative, than many
prescription and over the counter sleep aids if a sleeping medication
must be used for an extended period of time. Low-doses of melatonin are
usually sufficient to produce a
hypnotic
effect in most people. Higher doses do not appear to result in a
stronger effect, but instead appear to cause drowsiness for a longer
period of time.
Functions
When
eyes receive light from the sun, the pineal gland's production of
melatonin is inhibited and the hormones produced keep the human awake.
When the eyes do not receive light, melatonin is produced in the pineal
gland and the human becomes tired.
Circadian rhythm
In animals, melatonin plays an important role in the regulation of
sleep–wake cycles.
Human infants' melatonin levels become regular in about the third month
after birth, with the highest levels measured between midnight and 8:00
am. Human melatonin production decreases as a person ages. Also, as children become teenagers, the nightly schedule of melatonin release is delayed, leading to
later sleeping and waking times.
Antioxidant
Melatonin was first reported as a potent antioxidant and free radical scavenger in 1993. In vitro, melatonin acts as a direct scavenger of oxygen radicals and reactive nitrogen species including OH
•, O
2−•, and NO
•. In plants, melatonin works with other antioxidants to improve the overall effectiveness of each antioxidant. Melatonin has been proven to be twice as active as vitamin E, believed to be the most effective lipophilic antioxidant. Via
signal transduction through
melatonin receptors, melatonin promotes the
expression of antioxidant
enzymes such as
superoxide dismutase,
glutathione peroxidase,
glutathione reductase, and
catalase.
Melatonin occurs at high concentrations within
mitochondrial fluid which greatly exceed the
plasma concentration of melatonin.
Due to its capacity for free radical scavenging, indirect effects on
the expression of antioxidant enzymes, and its significant
concentrations within mitochondria, a number of authors have indicated
that melatonin has an important physiological function as a
mitochondrial antioxidant.
Immune system
While it is known that melatonin interacts with the
immune system, the details of those interactions are unclear. An
antiinflammatory
effect seems to be the most relevant. There have been few trials
designed to judge the effectiveness of melatonin in disease treatment.
Most existing data are based on small, incomplete trials. Any positive
immunological effect is thought to be the result of melatonin acting on
high-affinity receptors (MT1 and MT2) expressed in immunocompetent
cells. In preclinical studies, melatonin may enhance
cytokine production, and by doing this, counteract
acquired immunodeficiences. Some studies also suggest that melatonin might be useful fighting infectious disease including viral, such as
HIV, and bacterial infections, and potentially in the treatment of
cancer.
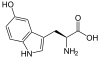
Biosynthesis
Overview of melatonin biosynthesis
In bacteria, protists, fungi, and plants, melatonin is
synthesized indirectly with tryptophan as an intermediate product of the
shikimate pathway. In these cells, synthesis starts with
D-erythrose 4-phosphate and
phosphoenolpyruvate, and in
photosynthetic cells with carbon dioxide. The rest of the synthesising reactions are similar, but with slight variations in the last two enzymes.
It has been hypothesized that melatonin is made in the mitochondria and chloroplasts.
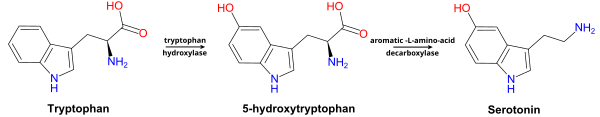
Mechanism
Mechanism of melatonin biosynthesis
In order to hydroxylate L-tryptophan, the cofactor
tetrahydrobiopterin
(THB) must first react with oxygen and the active site iron of
tryptophan hydroxylase. This mechanism is not well understood, but two
mechanisms have been proposed:
1. A slow transfer of one electron from the THB to O
2 could produce a
superoxide which could recombine with the THB
radical
to give 4a-peroxypterin. 4a-peroxypterin could then react with the
active site iron (II) to form an iron-peroxypterin intermediate or
directly transfer an oxygen atom to the iron.
2. O2 could react with the active site iron (II)
first, producing iron (III) superoxide which could then react with the
THB to form an iron-peroxypterin intermediate.
Iron (IV) oxide from the iron-peroxypterin intermediate is selectively attacked by a double bond to give a
carbocation
at the C5 position of the indole ring. A 1,2-shift of the hydrogen and
then a loss of one of the two hydrogen atoms on C5 reestablishes
aromaticity to furnish 5-hydroxy-L-tryptophan.
A decarboxylase with cofactor
pyridoxal phosphate (PLP) removes CO
2 from 5-hydroxy-L-tryptophan to produce 5-hydroxytryptamine. PLP forms an
imine with the amino acid derivative. The amine on the pyridine is
protonated and acts as an electron sink, enabling the breaking of the C-C bond and releasing CO
2.
Protonation of the amine from tryptophan restores the aromaticity of
the pyridine ring and then imine is hydrolyzed to produce
5-hydroxytryptamine and PLP.
It has been proposed that
histidine residue His122 of serotonin N-acetyl transferase is the catalytic residue that
deprotonates
the primary amine of 5-hydroxytryptamine, which allows the lone pair on
the amine to attack acetyl-CoA, forming a tetrahedral intermediate. The
thiol from
coenzyme A serves as a good
leaving group when
attacked by a general base to give N-acetylserotonin.
N-acetylserotonin is methylated at the hydroxyl position by S-adenosyl methionine (SAM) to produce
S-adenosyl homocysteine (SAH) and melatonin.
Regulation
In vertebrates, melatonin secretion is regulated by activation of the
beta-1 adrenergic receptor by
norepinephrine.
Norepinephrine elevates the intracellular cAMP concentration via
beta-adrenergic receptors and activates the cAMP-dependent protein
kinase A (PKA). PKA phosphorylates the penultimate enzyme, the
arylalkylamine N-acetyltransferase (AANAT). On exposure to (day)light,
noradrenergic stimulation stops and the protein is immediately destroyed
by
proteasomal proteolysis. Production of melatonin is again started in the evening at the point called the
dim-light melatonin onset.
Blue light, principally around
460–480 nm, suppresses melatonin biosynthesis,
proportional to the light intensity and length of exposure. Until
recent history, humans in temperate climates were exposed to few hours
of (blue) daylight in the winter; their fires gave predominantly yellow
light. The
incandescent light bulb widely used in the 20th century produced relatively little blue light. Light containing only wavelengths greater than 530 nm does not suppress melatonin in bright-light conditions.
Wearing glasses that block blue light in the hours before bedtime may
decrease melatonin loss. Use of blue-blocking goggles the last hours
before bedtime has also been advised for people who need to adjust to an
earlier bedtime, as melatonin promotes sleepiness.
Pharmacology
Pharmacodynamics
Pharmacokinetics
When used several hours before sleep according to the
phase response curve for melatonin in humans, small amounts (0.3 mg) of melatonin shift the circadian clock earlier, thus promoting earlier sleep onset and morning awakening.
Melatonin is rapidly absorbed and distributed, reaching peak plasma
concentrations after 60 minutes of administration, and is then
eliminated. Melatonin has a half life of 35–50 minutes.
In humans, 90% of orally administered exogenous melatonin is cleared in
a single passage through the liver, a small amount is excreted in
urine, and a small amount is found in saliva. The bioavalibility of melatonin is between 10 and 50%.
Melatonin is metabolised in the liver by cytochrome P450 enzyme
CYP1A2 to 6-hydroxymelatonin. Metabolites are conjugated with sulfuric
acid or glucuronic acid for excretion in the urine. 5% of melatonin is
excreted in the urine as the unchanged drug.
Some of the metabolites formed via the reaction of melatonin with a free radical include
cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), and N1-acetyl-5-methoxykynuramine (AMK).
History
In 1958,
dermatology professor
Aaron B. Lerner and colleagues at
Yale University, in the hope that a substance from the pineal might be useful in treating
skin diseases, isolated the hormone from
bovine pineal gland extracts and named it melatonin. In the mid-70s Lynch
et al. demonstrated that the production of melatonin exhibits a circadian rhythm in human pineal glands.
The discovery that melatonin is an antioxidant was made in 1993.
The first
patent for its use as a low-dose sleep aid was granted to
Richard Wurtman at
MIT in 1995. Around the same time, the hormone got a lot of press as a possible treatment for many illnesses.
The New England Journal of Medicine
editorialized in 2000: "With these recent careful and precise
observations in blind persons, the true potential of melatonin is
becoming evident, and the importance of the timing of treatment is
becoming clear."
Other animals
In vertebrates, melatonin is produced in darkness, thus usually at night, by the
pineal gland, a small endocrine gland
located in the center of the brain but outside the blood–brain barrier. Light/dark information reaches the
suprachiasmatic nuclei from retinal
photosensitive ganglion cells of the eyes
rather than the melatonin signal (as was once postulated). Known as
"the hormone of darkness", the onset of melatonin at dusk promotes
activity in
nocturnal (night-active) animals and sleep in diurnal ones including humans.
Many animals use the variation in duration of melatonin production each day as a seasonal clock. In animals including humans,
the profile of melatonin synthesis and secretion is affected by the
variable duration of night in summer as compared to winter. The change
in duration of secretion thus serves as a biological signal for the
organization of daylength-dependent (
photoperiodic) seasonal functions such as reproduction, behavior, coat growth, and camouflage
coloring in seasonal animals.
In seasonal breeders that do not have long gestation periods and that
mate during longer daylight hours, the melatonin signal controls the
seasonal variation in their sexual physiology, and similar physiological
effects can be induced by exogenous melatonin in animals including
mynah birds and hamsters. Melatonin can suppress
libido by inhibiting secretion of
luteinizing hormone and
follicle-stimulating hormone from the
anterior pituitary gland, especially in mammals that have a
breeding season when daylight hours are long. The reproduction of
long-day breeders is
repressed by melatonin and the reproduction of
short-day breeders is stimulated by melatonin.
During the night, melatonin regulates
leptin, lowering its levels.
Cetaceans have lost all the genes for melatonin synthesis as well as those for melatonin receptors. This is thought to be related to their
unihemispheric sleep pattern (one brain hemisphere at a time). Similar trends have been found in
sirenians.
Plants
Until its
identification in plants in 1987, melatonin was for decades thought to
be primarily an animal neurohormone. When melatonin was identified in
coffee extracts in the 1970s, it was believed to be a byproduct of the
extraction process. Subsequently, however, melatonin has been found in
all plants that have been investigated. It is present in all the
different parts of plants, including leaves, stems, roots, fruits, and
seeds, in varying proportions.
Melatonin concentrations differ not only among plant species, but also
between varieties of the same species depending on the agronomic growing
conditions, varying from picograms to several micrograms per gram.
Notably high melatonin concentrations have been measured in popular
beverages such as coffee, tea, wine, and beer, and crops including corn,
rice, wheat, barley, and oats. In some common foods and beverages, including coffee and walnuts,
the concentration of melatonin has been estimated or measured to be
sufficiently high to raise the blood level of melatonin above daytime
baseline values.
Although a role for melatonin as a plant hormone has not been
clearly established, its involvement in processes such as growth and
photosynthesis is well established. Only limited evidence of endogenous
circadian rhythms in melatonin levels has been demonstrated in some
plant species and no membrane-bound receptors analogous to those known
in animals have been described. Rather, melatonin performs important
roles in plants as a growth regulator, as well as environmental stress
protector. It is synthesized in plants when they are exposed to both
biological stresses, for example, fungal infection, and nonbiological
stresses such as extremes of temperature, toxins, increased
soil salinity, drought, etc.
Occurrence
Dietary supplement
Melatonin is categorized by the US
Food and Drug Administration (FDA) as a dietary supplement, and is sold over-the-counter in both the US and Canada. The FDA regulations applying to medications are not applicable to melatonin.
As melatonin may cause harm in combination with certain medications or
in the case of certain disorders, a doctor or pharmacist should be
consulted before making a decision to take melatonin. In many countries, melatonin is recognized as a
neurohormone and it cannot be sold over-the-counter.
Food products
Naturally-occurring melatonin has been reported in foods including tart cherries to about 0.17–13.46 ng/g, bananas and grapes, rice and cereals, herbs, plums, olive oil,
wine
and beer. When birds ingest melatonin-rich plant feed, such as rice,
the melatonin binds to melatonin receptors in their brains.
When humans consume foods rich in melatonin, such as banana, pineapple,
and orange, the blood levels of melatonin increase significantly.
As reported in the
New York Times in May 2011,
beverages and snacks containing melatonin were being sold in grocery
stores, convenience stores, and clubs. The FDA considered whether these
food products could continue to be sold with the label "dietary
supplements". On 13 January 2010, it issued a Warning Letter to
Innovative Beverage, creators of several beverages marketed as drinks,
stating that melatonin, while legal as a dietary supplement, was not
approved as a
food additive. A different company selling a melatonin-containing beverage received a warning letter in 2015.
Commercial availability
Immediate-release
melatonin is not tightly regulated in countries where it is available
as an over-the-counter medication. It is available in doses from less
than half a milligram to 5 mg or more. Immediate-release formulations
cause blood levels of melatonin to reach their peak in about an hour.
The hormone may be administered orally, as capsules, gummies, tablets,
or liquids. It is also available for use sublingually, or as
transdermal patches.
Formerly, melatonin was derived from animal pineal tissue, such
as bovine. It is now synthetic and does not carry a risk of
contamination or the means of transmitting infectious material.
Melatonin is the most popular over-the-counter sleep remedy in
the US, resulting in sales in excess of US $400 million during 2017.
Research
A bottle of melatonin tablets. Melatonin is available in timed-release and in liquid forms.
Various uses and effects of melatonin have been studied. A 2015 review of studies of melatonin in
tinnitus found the quality of evidence low, but not entirely without promise.
Headaches
Tentative evidence shows melatonin may help reduce some types of headaches including
cluster and
hypnic headaches.
Cancer
A 2013 review by the National Cancer Institutes found evidence for use to be inconclusive. A 2005 review of unblinded
clinical trials found a reduced rate of death, but that blinded and independently conducted randomized controlled trials are needed.
Protection from radiation
Both animal and human
studies have shown melatonin to protect against radiation-induced
cellular damage. Melatonin and its metabolites protect organisms from
oxidative stress by scavenging
reactive oxygen species which are generated during exposure.
Nearly 70% of biological damage caused by ionizing radiation is
estimated to be attributable to the creation of free radicals,
especially the
hydroxyl radical
that attacks DNA, proteins, and cellular membranes. Melatonin has been
described as a broadly protective, readily available, and orally
self-administered antioxidant that is without major known side effects.
Epilepsy
A
2016 review found no beneficial role of melatonin in reducing seizure
frequency or improving quality of life in people with epilepsy.
Secondary dysmenorrhoea
A 2016 review suggested no strong evidence of melatonin compared to placebo for dysmenorrhoea secondary to endometriosis.
Delirium
A 2016 review suggested no clear evidence of melatonin to reduce the incidence of delirium.
Gastroesophageal reflux disease
A 2011 review said melatonin is effective in relieving epigastric pain and heartburn.
Psychiatry
Melatonin might improve sleep in people with
autism. Children with autism have abnormal melatonin pathways and below-average physiological levels of melatonin. Melatonin supplementation has been shown to improve sleep duration, sleep onset latency, and night-time awakenings. However, many studies on melatonin and autism rely on self-reported levels of improvement and more rigorous research is needed.
While the packaging of melatonin often warns against use in
people under 18 years of age, available studies suggest that melatonin
is an efficacious and safe treatment for insomnia in people with
ADHD. However, larger and longer studies are needed to establish long-term safety and optimal dosing.
Melatonin in comparison to placebo is effective for reducing
preoperative anxiety in adults when given as premedication. It may be
just as effective as standard treatment with midazolam in reducing
preoperative anxiety. Melatonin may also reduce postoperative anxiety
(measured 6 hours after surgery) when compared to placebo.
Some supplemental melatonin users report an increase in vivid dreaming. Extremely high doses of melatonin increased
REM sleep time and dream activity in people both with and without
narcolepsy. Some evidence supports an
antidepressant effect.